

苯并[h]喹啉腙类化合物的合成及生物活性研究
收稿日期: 2018-05-17
修回日期: 2018-06-05
网络出版日期: 2018-07-16
基金资助
国家自然科学基金(Nos.21605039,21702051)、河南省高等学校重点科学研究计划(Nos.18A150009,17A350006)和河南师范大学青年科学基金(No.2016QK10)资助项目.
Synthesis and Biological Activity of Benzo[h]quinolinium Hydrazine Compounds
Received date: 2018-05-17
Revised date: 2018-06-05
Online published: 2018-07-16
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21605039, 21702051), the Key Scientific Research Project of Henan Provinc (Nos. 18A150009, 17A350006), the Henan Normal University Science Foundation for Young Scholars (No. 2016QK10)
时蕾 , 徐晶晶 , 毕晶晶 , 张志国 , 刘统信 , 杨晓岚 , 张贵生 . 苯并[h]喹啉腙类化合物的合成及生物活性研究[J]. 有机化学, 2018 , 38(11) : 3016 -3025 . DOI: 10.6023/cjoc201805038
Quinoline derivatives, aryl hydrazines and hydrazide compounds have significant biological activities. Benzo[h] quinoline derivatives were synthesized from 1-naphthylamine, substituted benzaldehyde and methyl pyruvate by Doebner-Miller reaction. After the ester was reduced, the corresponding aldehyde was obtained by oxidation. Benzo[h] quinolinium and quinoline hydrazide compounds were synthesized by reacting benzo[h] quinoline formaldehyde with hydrazine salts and hydrazides, respectively. The preliminary activity data showed that most of the compounds exhibited significant inhibitory activities against cycle protein 25B (CDC 25B) and protein tyrosine phosphatase PTP 1B.
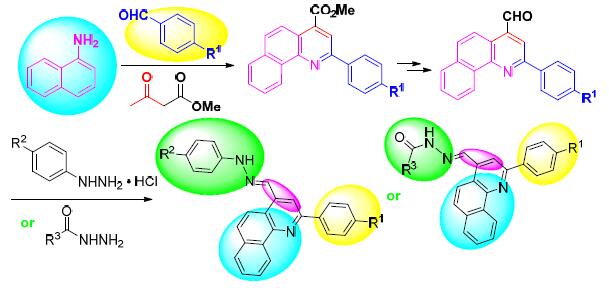
Key words: Benzo[h]quinoline derivatives; hydrazone; hydrazide; CDC 25B; PTP 1B; inhibitory activity
[1] Abner, E.; Stoszko, M.; Zeng, L.; Chen, H. C.; Izquierdo-Bouldstridge, A.; Konuma, T.; Zorita, E.; Fanunza, E.; Zhang, Q.; Mahmoudi, T.; Zhou, M. M.; Filion, G. J.; Jordan, A. J. Virol. 2018, 92, 10.
[2] Kwon, S.; Lee, Y.; Jung, Y.; Kim, J. H.; Baek, B.; Lim, B.; Lee, J.; Kim, I.; Lee, J. Eur. J. Med. Chem. 2018, 148, 116.
[3] Cai, Y.; Liu, H.; Chen, H. Chem. Biol. Drug Des. 2018, 91, 805.
[4] Baba, A.; Kawamura, N.; Makino, H. J. Med. Chem. 1996, 39, 5176.
[5] Chopra, R.; Chibale, K.; Singh, K. Eur. J. Med. Chem. 2018, 148, 39.
[6] Wang, W. Q.; Huang, L.; Cui, P.; Wu, Q. Y. Chin. J. Org. Chem. 2016, 36, 1065(in Chinese). (汪万强, 黄琳, 崔萍, 吴琼友, 有机化学, 2016, 36, 1065.)
[7] Men, Y.; Dong, J. H.; Wang, S.; Xu, X. X. Org. Lett. 2017, 19, 6712.
[8] Roberta, R. S.; Jose, M. F. S.; Bianca, C. C. Bioorg. Med. Chem. Lett. 2015, 25, 2309.
[9] Gross, D. J.; Weiss, L.; Reibstein, I. H. Int. Immunopharcol. 2001, 1, 115.
[10] Li, K.; Li, Y.; Zhou, D. Bioorg. Med. Chem. 2016, 24, 1889.
[11] Nagabhushana, N.; Jurupula, R.; Udayakumar, D. J. Fluorine Chem. 2016, 183, 59.
[12] Rita, C. C. C.; Wagner, A. M.; Tayara, P. S. Bioorg. Med. Chem. Lett. 2016, 26, 1881.
[13] Ravikumar, C.; Joe, I. H.; Jayakumar, V. S. Chem. Phys. Lett. 2008, 460, 552.
[14] Xiang, Y.; Tong, A. J.; Jin, P. Y; Ju, Y. Org. Lett. 2006, 8, 2863.
[15] Özkay, Y.; Tunali, Y.; Karaca, H.; IsIkdag, I. Eur. J. Med. Chem. 2010, 45, 3293.
[16] Du, H.; Fan. Y. J.; Yang, L.; Bao, X. P. Chin. J. Org. Chem. 2018, 38, 531(in Chinese). (杜欢, 范冶江, 杨岚, 鲍小平, 有机化学, 2018, 38, 531.)
[17] Liu, J. C.; Liu. Y. J.; He, H. W. Chin. J. Org. Chem. 2015, 35, 462(in Chinese). (刘建超, 刘勇军, 贺红武, 有机化学, 2015, 35, 462.)
/
| 〈 |
|
〉 |