

苯胺促进的异苯并呋喃酮类衍生物的合成
收稿日期: 2018-06-21
修回日期: 2018-07-16
网络出版日期: 2018-07-24
基金资助
国家自然科学基金(Nos.81430085,81773562,81703326)、国家重点研发计划(No.2016YFA0501800)、河南省科技攻关项目(No.182102310123)、中国博士后面上基金(No.2018M630840)、广东省新药筛选重点实验室开放课题(No.GDKLNDS-2018OF006)、河南省高等学校重点科研项目(No.18B350009)和郑州大学大学生创新创业训练计划(No.201810459101)资助项目.
Aniline-Promoted Synthesis of Isobenzofuranone Derivatives
Received date: 2018-06-21
Revised date: 2018-07-16
Online published: 2018-07-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81430085, 81773562, 81703326), the National Key Research Program of Proteins (No. 2016YFA0501800), the Scientific Program of Henan Province (No. 182102310123), the China Postdoctoral Science Foundation (No. 2018M630840), the Open Project of Guangdong Provincial Key Laboratory of New Drug Screening (No. GDKLNDS-2018OF006), the Key Research Program of Higher Education of Henan Province (No. 18B350009), and the Undergraduate Innovation and Entrepreneurship Training Program of Zhengzhou University (No. 201810459101).
袁硕 , 王四喜 , 陈锦杰 , 赵龙飞 , 余斌 , 刘宏民 . 苯胺促进的异苯并呋喃酮类衍生物的合成[J]. 有机化学, 2018 , 38(11) : 3009 -3015 . DOI: 10.6023/cjoc201806035
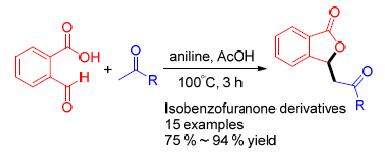
Isobenzofuranone derivatives are widely found in nature and have shown diverse biological activities. In this work, the aniline-promoted synthesis of isobenzofuranone derivatives starting from 2-carboxybenzaldehyde and substituted acetophenones under mild reaction conditions is reported. This method has broad substrate scope, high yields, and is operationally convenient, and therefore could serve as an attractive strategy for practical synthesis of isobenzofuranone derivatives.
Key words: isobenzofuranone derivatives; aniline; cascade reactions
[1] Dragan, A.; Jones, D. H.; Kennedy, A. R.; Tomkinson, N. C. Org. Lett. 2016, 18, 3086.
[2] (a) Rosengren, A. M.; Karlsson, B. C.; Nicholls, I. A. ACS Med. Chem. Lett. 2012, 3, 650.
(b) Matsumori, N.; Morooka, A.; Murata, M. J. Med. Chem. 2006, 49, 3501.
[3] Sueki, S.; Wang, Z. J.; Kuninobu, Y. Org. Lett. 2016, 18, 304.
[4] (a) Yu, B.; Wang, S. Q.; Qi, P. P.; Yang, D. X.; Tang, K.; Liu, H. M. Eur. J. Med. Chem. 2016, 124, 350.
(b) Shi, X. J.; Yu, B.; Wang, J. W.; Qi, P. P.; Tang, K.; Huang, X.; Liu, H. M. Sci. Rep. 2016, 6, 31607.
[5] Tu, F. X.; Pang, Q. Y.; Huang, T. T.; Zhao, Y.; Liu, M. X.; Chen, X. Med. Sci. Monit. 2017, 23, 4004.
[6] (a) Kim, H.; Swamy, K. M. K.; Kwon, N.; Kim, Y.; Park, S.; Yoon, J. ChemPhysChem. 2017, 18, 1752.
(b) Liang, B.; Zou, J.; Su, J. Pharmacoepidemiol. Drug Saf. 2015, 24, 555.
[7] (a) Butkevich, A. N.; Ta, H.; Ratz, M.; Stoldt, S.; Jakobs, S.; Belov, V. N.; Hell, S. W. ACS Chem. Biol. 2018, 13, 475.
(b) Zhu, X. Q.; Zhou, J.; Wang, C. H.; Li, X. T.; Jing, S. J. Phys. Chem. B 2011, 115, 3588.
(c) Rabiee, A.; Ebrahim-Habibi, A.; Navidpour, L.; Morshedi, D.; Ghasemi, A.; Sabbaghian, M.; Nemati-Lay, M.; Nemat-Gorgani, M. Chem. Biol. Drug Des. 2011, 78, 659.
(d) Hadj-Esfandiari, N.; Navidpour, L.; Shadnia, H.; Amini, M.; Samadi, N.; Faramarzi, M. A.; Shafiee, A. Bioorg. Med. Chem. Lett. 2007, 17, 6354.
[8] (a) Yaremenko, A. G.; Shelyakin, V. V.; Volochnyuk, D. M.; Rusanov, E. B.; Grygorenko, O. O. Tetrahedron Lett. 2013, 54, 1195.
(b) Akagi, Y.; Yamada, S. I.; Etomi, N.; Kumamoto, T.; Nakanishi, W.; Ishikawa, T. Tetrahedron Lett. 2010, 51, 1338.
(c) Karmakar, R.; Pahari, P.; Mal, D. Tetrahedron Lett. 2009, 50, 4042.
[9] Lee, D. Y.; Cho, C. S.; Jiang, L. H.; Wu, X.; Shim, S. C.; Oh, D. H. Synth. Commun. 2006, 27, 3449.
[10] Sahoo, S. C.; Nath, U.; Pan, S. C. Eur. J. Org. Chem. 2017, 2017, 4434.
[11] Goncalves, C. J.; Lenoir, A. S.; Padaratz, P.; Correa, R.; Niero, R.; Cechinel-Filho, V.; Campos B. F. Eur. J. Med. Chem. 2012, 56, 120.
[12] (a) Sangshetti, J. N.; Ansari, S. A. M. K.; Shinde, D. B. Chin. Chem. Lett. 2011, 22, 163.
(b) da Silva Maia, A. F.; Siqueira, R. P.; de Oliveira, F. M.; Ferreira, J. G.; da Silva, S. F.; Caiuby, C. A. D.; de Oliveira, L. L.; de Paula, S. O.; Souza, R. A. C.; Guilardi, S.; Bressan, G. C.; Teixeira, R. R. Bioorg. Med. Chem. Lett. 2016, 26, 2810.
[13] (a) Heravi, M. M.; Rasmi, V.; Bamoharram, F. F.; Sadjadi, S.; Fotouhi, L.; Sadjadi, S.; Bakavoli, M. Synth. Commun. 2009, 39, 4109.
(b) Rastegari, F.; Mohammadpoor-Baltork, I.; Khosropour, A. R.; Tangestaninejad, S.; Mirkhani, V.; Moghadam, M. RSC Adv. 2015, 5, 15274.
[14] (a) Zambre, S. S.; Darandale, S. N.; Sangshetti, J. N.; Shinde, D. B. Arabian J. Chem. 2016, 9, 1416.
(b) Landge, S. M.; Berryman, M.; Török, B. Tetrahedron Lett. 2008, 49, 4505.
[15] Kore, R.; Srivastava, R. Catal. Commun. 2011, 12, 1420.
[16] Palillero-Cisneros, A.; Bedolla-Medrano, M.; Ordóñez, M. Tetrahedron 2018, 74, 4174.
[17] Bassin, J. P.; Anagani, B.; Benham, C.; Goyal, M.; Hashemian, M.; Gerhard, U. Molecules 2016, 21, 967.
[18] (a) Yu, C. G.; Huang, H.; Li, X. M.; Zhang, Y. T.; Li, H.; Wang, W. Chem. Eur. J. 2016, 22, 9240.
(b) Balaraman, K.; Ding, R. S.; Wolf, C. Adv. Synth. Catal. 2017, 359, 4165.
/
| 〈 |
|
〉 |