

含苯并咪(噻)唑环的β-咔啉衍生物的合成与杀菌活性
收稿日期: 2018-05-29
修回日期: 2018-07-07
网络出版日期: 2018-08-22
基金资助
新疆维吾尔自治区研究生科研创新项目基金(No.XJGRI2017045)、国家大学生创新创业训练计划(No.201810759055)、教育部长江学者和创新团队发展计划(No.IRT15R46)及石河子大学长江学者研究(No.CJXZ201601)资助项目.
Synthesis and Fungicidal Evaluation of Novel β-Carboline-Benzimidazole and β-Carboline-Benzothiazole Hybrids
Received date: 2018-05-29
Revised date: 2018-07-07
Online published: 2018-08-22
Supported by
Project supported by the Scientific Research Innovation Project in Xinjiang Uygur Autonomous Region (No. XJGRI2017045), the National Students Innovation and Entrepreneuship Training Program (No. 201810759055), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT15R46), and the Yangtze River Scholar Research Project of Shihezi University (No. CJXZ201601).
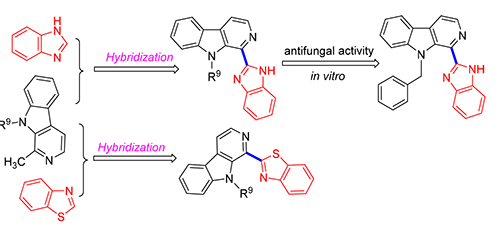
为了寻找结构新颖的活性分子,采用活性结构拼接方法,合成了14个具有β-咔啉和苯并咪唑或者同它的生物电子等排体苯并噻唑连接的新分子,其结构经1H NMR、13C NMR和HRMS确证.进一步测试了这些化合物对棉花立枯丝核菌、棉花枯萎病原菌、葡萄灰霉病原菌、向日葵菌核和油菜菌核的杀菌活性.离体杀菌活性测定结果表明,在50 μg·mL-1时,多数目标化合物对供试5种病原菌表现出一定的抑制活性,但活性均不高.其中β-咔啉环9位为乙基、苄基、3-氯苄基的苯并咪唑衍生物(4a、4c和4e)对向日葵菌核有较高抑制率,而1-苯并咪唑-9-乙基-β-咔啉(4a)、1-苯并咪唑-9-丁基-β-咔啉(4b)、1-苯并咪唑-9-苄基-β-咔啉(4c)、1-苯并咪唑-9-(2,3,4,5,6-五氟苄基)-β-咔啉(4f)、1-苯并噻唑-9-(3-氯苄基)-β-咔啉(5e)和1-苯并噻唑-9-(2,3,4,5,6-五氟苄基)-β-咔啉(5f)则对油菜菌核表现出令人满意的抗菌活性.其中化合物4c对大多数的测试菌株表现出广谱活性.
霍新玉 , 李文斌 , 张博雅 , 陈晓飞 , 周月婷 , 张洁 , 韩小强 , 代斌 . 含苯并咪(噻)唑环的β-咔啉衍生物的合成与杀菌活性[J]. 有机化学, 2018 , 38(12) : 3356 -3362 . DOI: 10.6023/cjoc201805053
In order to discover novel compounds with biological activities, new molecular hybrids combining benzimidazole or its bioisostere benzothiazole with β-carboline were synthesized. The benzimidazole or benzothiazole scaffold was linked at position-1 with β-carboline which was further characterized by 1H NMR, 13C NMR and HRMS. All of the target compounds were evaluated in vitro for their antifungal activity against Rhizoctorzia solani, Fusarium oxysporum, Botrytis cinerea Pers., sunflower sclerotinia rot and rape sclerotinia rot by mycelia growth inhibition assay at 50 μg·mL-1. The preliminary results showed that most compounds exhibit mild inhibiting effect against all the tested strains. Among them, 1-(1H-benzo[d]-imida-zol-2-yl)-9-ethyl-β-carboline (4a), 1-(1H-benzo[d]imidazol-2-yl)-9-benzyl-β-carboline (4c) and 1-(1H-benzo[d]imidazol-2-yl)-9-(3-chlorobenzyl)-β-carboline (4e) showed satisfactory antifungal activity against sunflower sclerotinia rot, 1-(1H-benzo[d]-imidazol-2-yl)-9-ethyl-β-carboline (4a), 1-(1H-benzo[d]imidazol-2-yl)-9-n-butyl-β-carboline (4b), 1-(1H-benzo[d]imidazol-2-yl)-9-benzyl-β-carboline (4c), 1-(1H-benzo[d]imidazol-2-yl)-9-((perfluorophenyl)methyl)-β-carboline (4f), 1-(benzo[d]-thiazol-2-yl)-9-(3-chlorobenzyl)-β-carboline (5e) and 1-(benzo[d]thiazol-2-yl)-9-((perfluorophenyl)methyl)-β-carboline (5f) displayed excellent fungicidal activity against rape sclerotinia rot. Specifically, compound 4c exhibited broad-spectrum fungicidal activity against most of the tested fungi.

Key words: β-carboline; benzimidazole; benzothiazole; antifungal activity
[1] Qiu, D.-W.; Dong, Y.-J.; Zhang, Y.; Li, S.-P.; Shi, F.-C. Mol Plant Microbe Interact. 2017, 30, 355.
[2] Xu, W.-M.; He, J.; He, M.; Han, F.-F.; Chen, X.-H.; Pan, Z.-X.; Wang, J.; Tong, M.-G. Molecules 2011, 16, 9129.
[3] Pavela, R.; Vrchotová, N. Ind. Crops Prod. 2013, 43, 33.
[4] Meester, C. D. Mutat. Res. 1995, 339, 139.
[5] Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P.; Phil, S. J. Med. Chem. 1982, 25, 1081.
[6] Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Eur. J. Integr. Med. 2017, 9, 91.
[7] Asgarpanah, J.; Ramezanloo, F. Afr. J. Pharm. Pharmacol. 2012, 6, 1573.
[8] Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. Bioorg. Med. Chem. 1999, 7, 1223.
[9] Wang, Y.-H.; Tang, J.-G.; Wang, R.-R.; Yang, L.-M.; Dong, Z.-J.; Du, L.; Shen, X.; Liu, J.-K.; Zheng, Y.-T. Biochem. Biophys. Res. Commun. 2007, 355, 1091.
[10] Zhang, Z.-J.; Zhang, J.-J.; Jiang, Z.-Y.; Zhong, G.-H. Molecules 2017, 22, 1811.
[11] Nenaah, G. J. Stored Prod. Res. 2011, 47, 255.
[12] Abbasipour, H.; Mahmoudvand, M.; Rastegar, F.; Basij, M. Bull. Insectol. 2010, 63, 259.
[13] Song, H.-J.; Liu, Y.-X.; Liu, Y.-X.; Wang, Q.-M. J. Agric. Food Chem. 2014, 62, 1010.
[14] Huang, Y.-Q.; Liu, Y.-X.; Liu, Y.-X.; Song, H.-J.; Wang, Q.-M. Bioorg. Med. Chem. 2016, 24, 462.
[15] Li, Z.-B.; Chen, S.-H.; Zhu, S.-W.; Luo, J.-J.; Zhang, Y.-M.; Weng, Q.-F. Molecules 2015, 20, 13941.
[16] Huo, X.-Y.; Guo, L.; Wei, Y.-T.; Zhang, J.; Han, X.-Q. Agrochemicals 2018, 57, 3(in Chinese). (霍新玉, 郭亮, 韦玥婷, 张洁, 韩小强, 农药, 2018, 57, 3.)
[17] Patil, S. A.; Patil, S. A.; Patil, R. Chem. Biol. Drug Des. 2017, 89, 639.
[18] El-Gohary, N. S.; Shaaban, M. I. Eur. J. Med. Chem. 2017, 131, 255.
[19] Alasmary, F. A. S.; Snelling, A. M.; Zain, M. E.; Alafeefy, A. M.; Awaad, A. S.; Karodia, N. Molecules 2015, 20, 15206.
[20] Gray, L. E.; Ostby, J.; Linder, R.; Goldman, J.; Rehnberg, G.; Cooper, R. Fundam. Appl. Toxicol. 1990, 15, 281.
[21] Feng, J.-Y.; Hu, Y.-X.; Grant, E.; Lu, X.-N. Food Chem. 2018, 239, 816.
[22] Zhao, S.-Z.; Zhao, L.-Y.; Zhang, X.-Q.; Liu, C.-C.; Hao, C.-Z.; Xie, H.-L.; Sun, B.; Zhao, D.-M.; Cheng, M.-S. Eur. J. Med. Chem. 2016, 123, 514.
[23] Chugunova, E.; Boga, C.; Sazykin, I.; Cino, S.; Micheletti, G.; Mazzanti, A.; Sazykina, M.; Burilov, A.; Khmelevtsova, L.; Kostina, N. Eur. J. Med. Chem. 2015, 93, 349.
[24] Tomlin, C. E. The Pesticide Manual, 11th ed., The British Crop Protection Council, Surrey, 1997, p. 463.
[25] Smith, A. E. J. Agric. Food Chem. 1985, 33, 483.
[26] Guo, L.; Fan, W.-X.; Chen, W.; Ma, Q.; Cao, R.-H. J. Chin. Pharm. Sci. 2015, 24, 801.
[27] (a) Guo, L.; Fan, W.-X.; Chen, X.-M.; Ma, Q.; Cao, R.-H. Chin. J. Org. Chem. 2013, 33, 332(in Chinese). (郭亮, 范文玺, 陈雪梅, 马芹, 曹日晖, 有机化学, 2013, 33, 332.)
(b) Guo, L.; Xie, J.-W.; Fan, W.-X.; Chen, W.; Dai, B.; Ma, Q. Chin. J. Org. Chem. 2017, 37, 1741(in Chinese). (郭亮, 谢建伟, 范文玺, 陈伟, 代斌, 马芹, 有机化学, 2017, 37, 1741.)
[28] Chen, W.; Zhang, G.-X.; Guo, L.; Fan, W.-X.; Ma, Q.; Zhang, X.-D.; Du, R.-L.; Cao, R.-H. Eur. J. Med. Chem. 2016, 124, 249.
[29] Yuan, X.-Y.; Zhang, L.; Han, X.-Q.; Zhou, Z.-Y.; Du, S.-J.; Wan, C.; Yang, D.-Y.; Qin, Z.-H. Chin. J. Org. Chem. 2014, 34, 170(in Chinese). (袁小勇, 张鹭, 韩小强, 周子原, 杜士杰, 万川, 杨冬燕, 覃兆海, 有机化学, 2014, 34, 170.)
[30] Pesticides Guidelines for Laboratory Bioactivity Tests:Part 2:Petri Plate Test for Determining Fungicide Inhibition of Mycelial Growth:NY/T1156.2-2006, China Agriculture Press, Beijing, 2006(in Chinese). (农药室内生物测定试验准则杀菌剂第2部分:抑制病原真菌菌丝生长试验平皿法:NY/T1156.2-2006. 中国农业出版社, 北京, 2006.)
/
| 〈 |
|
〉 |