

基于杂环的不对称插烯Mannich反应及其在生物碱合成中的应用进展
收稿日期: 2018-06-03
修回日期: 2018-08-22
网络出版日期: 2018-08-22
基金资助
国家重点研发计划(No.2017YFA0207302)、国家自然科学基金(Nos.21332007,21472153)、教育部长江学者和创新团队发展计划、中央高校基本科研业务费专项资金(Nos.20720170092,20720180024)和福建省自然科学基金(No.2017J01021)资助项目.
Progress in Heterocycles-Based Asymmetric Vinylogous Mannich Reactions and Applications to the Synthesis of Alkaloids
Received date: 2018-06-03
Revised date: 2018-08-22
Online published: 2018-08-22
Supported by
Project supported by the National Key R&D Program of China (No. 2017YFA0207302), the National Natural Science Foundation of China (Nos. 21332007, 21472153), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) of Ministry of Education, the Chinese Universities Scientific Fund (Nos. 20720170092, 20720180024) and the Natural Science Foundation of Fujian Province of China (No. 2017J01021).
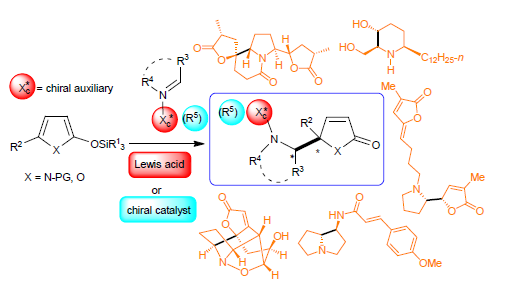
基于杂环(α,β/β,γ-不饱和γ-内酯,α,β-不饱和γ-内酰胺)的插烯Mannich反应在构建新C-C键的同时形成含邻氨基醇片段的α,β-不饱和γ-内酯和含邻二胺片段的α,β-不饱和γ-内酰胺结构单元,是构建含氧、含氮杂环及合成生物碱的重要合成砌块,具有广泛的应用价值.对2011年以来基于杂环的不对称插烯Mannich反应研究进展进行综述,涵盖手性辅助基诱导的插烯Mannich反应、金属-手性配体络合物和有机小分子催化的不对称插烯Mannich反应及其在复杂生物碱合成中的应用.文中也对相关方法的局限进行了分析.
叶剑良 , 黄培强 . 基于杂环的不对称插烯Mannich反应及其在生物碱合成中的应用进展[J]. 有机化学, 2018 , 38(9) : 2215 -2230 . DOI: 10.6023/cjoc201806005
Heterocycles (α,β/β,γ-unsaturated-γ-lactones, α,β-unsaturated-γ-lactams)-based vinylogous Mannich reactions (VMR) constitute a class of effective C-C bond formation approach to install vicinal aminol-containing α,β-unsaturated-lactones and vicinal diamine-containing α,β-unsaturated-γ-lactams. Possessing multiple functionalities, the latters are versatile building blocks for the synthesis of O-heterocycles, N-heterocycles and the synthesis of alkaloids. The progresses of the asymmetric vinylogous Mannich reactions of silyloxy pyrroles and silyloxy furans from 2011 to mid-2018 are summarized. The methods are organized according to chiral auxiliary-induced asymmetric VMRs, asymmetric VMRs catalyzed by metal-chiral ligand complex or organocatalyst, and the applications of the aymmetric VMRs to the syntheses of complex alkaloids. Some limitations of the developed heterocycles-based VMRs are also briefly discussed.

[1] Liu, Y.; Wu, Q.; Yin, D.; Li, D. Chin. J. Org. Chem. 2016, 36, 927(in Chinese). (刘玉婷, 吴倩倩, 尹大伟, 李荻扬, 有机化学, 2016, 36, 927.)
[2] (a) Wehlauch, R.; Gademann, K. Asian J. Org. Chem. 2017, 6, 1146.
(b) Martin, S. F. Adv. Heterocycl. Chem. 2013, 110, 73.
[3] (a) Zhang, Q.; Liu, X.; Feng, X. Curr. Org. Synth. 2013, 10, 764.
(b) Casiraghi, G.; Battistini, L.; Curti, C.; Rassu, G.; Zanardi, F. Chem. Rev. 2011, 111, 3076.
(c) Casiraghi, G.; Zanardi, F.; Battistini, L.; Rassu, G. Synlett 2009, 1525.
(d) Martin, S. F. Acc. Chem. Res. 2002, 35, 895.
(e) Bur, S. K.; Martin, S. F. Tetrahedron 2001, 57, 3221.
[4] Sartori, A.; Dell'Amico, L.; Curti, C.; Battistini, L.; Pelosi, G.; Rassu, G.; Casiraghi, G.; Zanardi, F. Adv. Synth. Catal. 2011, 353, 3278.
[5] Sartori, A.; Dell'Amico, L.; Battistini, L.; Curti, C.; Rivara, S.; Pala, D.; Kerry, P. S.; Pelosi, G.; Casiraghi, G.; Rassu, G.; Zanardi, F. Org. Biomol. Chem. 2014, 12, 1561.
[6] Robak, M. T.; Herbage, M. A.; Ellman, J. A. Chem. Rev. 2010, 110, 3600.
[7] Ruan, S.-T.; Luo, J.-M.; Du, Y.; Huang, P.-Q. Org. Lett. 2011, 13, 4938.
[8] Bur, S. K.; Martin, S. F. Org. Lett. 2000, 2, 3445.
[9] Harding, K. E.; Southard, J. M. Tetrahedron:Asymmetry 2005, 16, 1845.
[10] Guo, L.-D.; Liang, P.; Zheng, J.-F.; Huang, P.-Q. Eur. J. Org. Chem. 2013, 2230.
[11] Liu, R.-C.; Wei, J.-H.; Wei, B.-G.; Lin, G.-Q. Tetrahedron:Asymmetry 2008, 19, 2731.
[12] Altenbach, H.-J.; Himmeldirk, K. Tetrahedron:Asymmetry 1995, 6, 1077.
[13] Banba, Y.; Abe, C.; Nemoto, H.; Kato, A.; Adachi, I.; Takahata, H. Tetrahedron:Asymmetry 2001, 12, 817.
[14] Nadin, A.; Sánchez López, J. M.; Neduvelil, J. G.; Thomas, S. R. Tetrahedron 2001, 57, 1861.
[15] Rao, V. U. B.; Jadhav, A. P.; Garad, D.; Singh, R. P. Org. Lett. 2014, 16, 648.
[16] Shi, Y.-H.; Wang, Z.; Shi, Y.; Deng, W.-P. Tetrahedron 2012, 68, 3649.
[17] Liu, L.-J.; Chen, L.-J.; Li, P.; Li, X.-B.; Liu, J.-T. J. Org. Chem. 2011, 76, 4675.
[18] Liu, L.-J.; Liu, J.-T. Tetrahedron 2014, 70, 1236.
[19] Yu, J.; Miao, Z.; Chen, R. Org. Biomol. Chem. 2011, 9, 1756.
[20] Tamura, O.; Takeda, K.; Mita, N.; Sakamoto, M.; Okamoto, I.; Morita, N.; Ishibashi, H. Org. Biomol. Chem. 2011, 9, 7411.
[21] Degiorgis, F.; Lombardo, M.; Trombini, C. Tetrahedron 1997, 53, 11721.
[22] Garner, P.; Park, J. M. J. Org. Chem. 1990, 55, 3772.
[23] Yuan, Z.-L.; Jiang, J.-J.; Shi, M. Tetrahedron 2009, 65, 6001.
[24] Zhao, Q.-Y.; Yuan, Z.-L.; Shi, M. Tetrahedron:Asymmetry 2010, 21, 943.
[25] Zhao, Q.-Y.; Yuan, Z.-L.; Shi, M. Adv. Synth. Catal. 2011, 353, 637.
[26] Zheng, L.-S.; Li, L.; Yang, K.-F.; Zheng, Z.-J.; Xiao, X.-Q.; Xu, L.-W. Tetrahedron 2013, 69, 8777.
[27] Hayashi, M.; Sano, M.; Funahashi, Y.; Nakamura, S. Angew. Chem., Int. Ed. 2013, 52, 5557.
[28] (a) Carswell, E. L.; Snapper, M. L.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2006, 45, 7230.
(b) Mandai, H.; Mandai, K.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2008, 130, 17961.
(c) Wieland, L. C.; Vieira, E. M.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2009, 131, 570.
[29] Curti, C.; Battistini, L.; Ranieri, B.; Pelosi, G.; Rassu, G.; Casiraghi, G.; Zanardi, F. J. Org. Chem. 2011, 76, 2248.
[30] Silverio, D. L.; Fu, P.; Carswell, E. L.; Snapper, M. L.; Hoveyda, A. H. Tetrahedron Lett. 2015, 56, 3489.
[31] Rainoldi, G.; Sacchetti, A.; Silvani, A.; Lesma, G. Org. Biomol. Chem. 2016, 14, 7768.
[32] Zhou, L.; Lin, L.; Ji, J.; Xie, M.; Liu, X.; Feng, X. Org. Lett. 2011, 13, 3056.
[33] Guo, Y.-L.; Bai, J.-F.; Peng, L.; Wang, L.-L.; Jia, L.-N.; Luo, X.-Y.; Tian, F.; Xu, X.-Y.; Wang, L.-X. J. Org. Chem. 2012, 77, 8338.
[34] Guo, Y.; Zhang, Y.; Qi, L.; Tian, F.; Wang, L. RSC Adv. 2014, 4, 27286.
[35] Yin, L.; Takada, H.; Kumagai, N.; Shibasaki, M. Angew. Chem., Int. Ed. 2013, 52, 7310.
[36] Nakamura, S.; Yamaji, R.; Hayashi, M. Chem.-Eur. J. 2015, 21, 9615.
[37] Trost, B. M.; Gnanamani, E.; Tracy, J. S.; Kalnmals, C. A. J. Am. Chem. Soc. 2017, 139, 18198.
[38] Wang, Z.-H.; You, Y.; Chen, Y.-Z.; Xu, X.-Y.; Yuan, W.-C. Org. Biomol. Chem. 2018, 16, 1636.
[39] Hitotsuyanagi, Y.; Takeda, E.; Fukaya, H.; Takeya, K. Tet-rahedron Lett. 2008, 49, 7376.
[40] Wang, A.-E.; Huang, P.-Q. Pure Appl. Chem. 2014, 86, 1227.
[41] Tuo, S.-C.; Ye, J.-L.; Wang, A.-E.; Huang, S.-Y.; Huang, P.-Q. Org. Lett. 2011, 13, 5270.
[42] Liu, X.-K.; Ye, J.-L.; Ruan, Y.-P.; Li, Y.-X.; Huang, P.-Q. J. Org. Chem. 2013, 78, 35.
[43] (a) Hanessian, S.; McNaughton-Smith, G. Bioorg. Med. Chem. Lett. 1996, 6, 1567.
(b) Rassu, G.; Carta, P.; Pinna, L.; Battistini, L.; Zanardi, F.; Acquotti, D.; Casiraghi, G. Eur. J. Org. Chem. 1999, 1395.
[44] Miyatake-Ondozabal, H.; Bannwart, L. M.; Gademann, K. Chem. Commun. 2013, 49, 1921.
[45] Wehlauch, R.; Grendelmeier, S. M.; Miyatake-Ondozabal, H.; Sandtorv, A. H.; Scherer, M.; Gademann, K. Org. Lett. 2017, 19, 548.
[46] Ye, J.-L.; Zhang, Y.-F.; Liu, Y.; Zhang, J.-Y.; Ruan, Y.-P.; Huang, P.-Q. Org. Chem. Front. 2015, 2, 697.
[47] Oudeyer, S.; Dudot, B.; Royer, J. Heterocycles 2005, 65, 823.
[48] Ye, J.-L.; Chen, H.; Zhang, Y.-F.; Huang, P.-Q. Org. Chem. Front. 2016, 3, 683.
[49] Ye, J.-L.; Liu, Y.; Yang, Z.-P.; Huang, P.-Q. Chem. Commun. 2016, 52, 561.
[50] Ye, J.-L.; Liu, Y.; Zhang, Y.-F.; Yang, Z.-P.; Huang, P.-Q. Synthesis 2016, 48, 1684.
[51] Yoritate, M.; Takahashi, Y.; Tajima, H.; Ogihara, C.; Yokoyama, T.; Soda, Y.; Oishi, T.; Sato, T.; Chida, N. J. Am. Chem. Soc. 2017, 139, 18386.
[52] Zhou, T.; Gao, J.; Liu, G.; Guan, X.; An, D.; Zhang, S.; Zhang, G. Synlett 2018, 29, 2006.
/
| 〈 |
|
〉 |