

铜催化的1,3-二羰基化合物、甲醇和乙酸铵的串联氧化环化合成2,3,5,6-四取代吡啶
收稿日期: 2018-07-01
修回日期: 2018-07-12
网络出版日期: 2018-08-22
基金资助
国家自然科学基金(No.21502177)、河南省科技攻关计划(No.182102310903)、郑州轻工业学院博士研究基金(No.2014BSJJ032)和郑州轻工业学院青年骨干教师资助项目.
Synthesis of 2,3,5,6-Tetrasubstituted Pyridines via Copper-Catalyzed Domino Oxidative Annulation of 1,3-Dicarbonyl Compounds with Methanol and Ammonium Acetate
Received date: 2018-07-01
Revised date: 2018-07-12
Online published: 2018-08-22
Supported by
Project supported by the National Natural Science Foundation of China (No. 21502177), the Scientific and Technological Breakthrough Plan of Henan Province (No. 182102310903), the Doctoral Research Foundation of Zhengzhou University of Light Industry (No. 2014BSJJ032), and the Grants Program of Youth Backbone Teachers Training Object of Zhengzhou University of Light Industry.
闫溢哲 , 李政 , 崔畅 , 刘延奇 . 铜催化的1,3-二羰基化合物、甲醇和乙酸铵的串联氧化环化合成2,3,5,6-四取代吡啶[J]. 有机化学, 2018 , 38(12) : 3381 -3385 . DOI: 10.6023/cjoc201807003
A copper-catalyzed oxidative formal cycloaddition of 1,3-dicarbonyl compounds, methanol and ammonium acetate was first demonstrated, affording symmetrical 2,3,5,6-tetrasubstituted pyridines in moderate to excellent yields. Methanol was employed as the carbon synthon as well as the reaction solvent. The preliminary mechanistic studies revealed that the reaction underwent a radical pathway and the C(sp3)-H bond cleavage of methanol was the rate-determining step. This method is operationally simple and environmentally friendly.
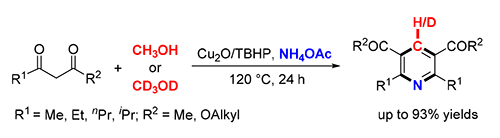
Key words: copper; oxidative annulation; pyridine; methanol
[1] (a) Roughley, S. D.; Jordan, A. M. J. Med. Chem. 2011, 54, 3451.
(b) Carey, J. S.; Laffan, D.; Thomson, C.; Williams, M. T. Org. Biomol. Chem. 2006, 4, 2337.
(c) Michael, J. P. Nat. Prod. Rep. 2005, 22, 627.
[2] Henry, G. D. Tetrahedron 2004, 60, 6043.
[3] (a) Xia, J.-J.; Wang, G.-W. Synthesis 2005, 2379.
(b) Litvic, M.; Cepanec, L.; Filipan, M.; Kos, K.; Bartolincic, A.; Druskovic, V.; Tibi, M. M.; Vinkovic, V. Heterocycles 2005, 65, 23.
(c) Nasr-Esfahani, M.; Karami, B.; Behzadi, M. J. Heterocycl. Chem. 2009, 46, 931.
(d) Xia, J.; Zhang, K.; Ju, J. Chin. J. Org. Chem. 2009, 29, 1849(in Chinese). (夏静静, 张克华, 鞠俊, 有机化学, 2009, 29, 1849).
(e) Chen, S.; Hossain, M. S.; Foss, F. W. ACS Sustainable Chem. Eng. 2013, 1, 1045.
(f) Abdel-Mohsen, H. T.; Conrad, J.; Beifuss, U. Green Chem. 2012, 14, 2686.
[4] Chang, L.; Lai, J.; Yuan, G. Chin. J. Chem. 2016, 34, 887.
[5] Yan, Y.; Li, H.; Li, Z.; Niu, B.; Shi, M.; Liu, Y. J. Org. Chem. 2017, 82, 8628.
[6] (a) Guo, S.-R.; Kumar, P. S.; Yang, M. Adv. Synth. Catal. 2017, 359, 2.
(b) Lakshman, M. K.; Vuram, P. K. Chem. Sci. 2017, 8, 5845.
[7] (a) Tang, L.; Guo, X.; Yang, Y.; Zha, Z.; Wang, Z. Chem. Commun. 2014, 50, 6145.
(b) Zhang, W; Guo, F.; Wang, F.; Zhao, N.; Liu, L.; Li, J.; Wang, Z. Org. Biomol. Chem. 2014, 12, 5752.
(c) Tang, L.; Yang, Y.; Wen, L.; Zhang, S.; Zha, Z.; Wang, Z. Org. Chem. Front. 2015, 2, 114.
(d) You, Q.; Wang, F.; Wu, C.; Shi, T.; Min, D.; Chen, H.; Zhang, W. Org. Biomol. Chem. 2015, 13, 6723.
(e) Tiwari, A. R.; T. A.; Bhanage, B. M. Org. Biomol. Chem. 2015, 13, 10973.
(f) Li, J.; Zhang, J.; Yang, H.; Gao, Z.; Jiang, G. J. Org. Chem. 2017, 82, 765.
(g) Guan, Q.; Sun, Q.; Wen, L.; Zha, Z.; Yang, Y.; Wang, Z. Org. Biomol. Chem. 2018, 16, 2088.
[8] (a) Yan, Y.; Zhang, Y.; Feng, C.; Zha, Z.; Wang, Z. Angew. Chem., Int. Ed. 2012, 51, 8077.
(b) Yan, Y.; Li, Z.; Li, H.; Cui, C.; Shi, M.; Liu, Y. Org. Lett. 2017, 19, 6228.
[9] (a) Zhang, L.; Fan, F.; Yang, J.; Liu, Z. Org. Lett. 2014, 16, 3396.
(b) Xu, Z.; Hang, Z.; Chai, L.; Liu, Z. Org. Lett. 2016, 18, 4662.
[10] (a) Miyamura, H.; Maehata, K.; Kobayashi, S. Chem. Commun. 2010, 46, 8052.
(b) Bai, C.-B.; Wang, N.-X.; Wang, Y.-J.; Lan, X.-W.; Xing, Y.; Wen, J.-L. RSC Adv. 2015, 5, 100531.
/
| 〈 |
|
〉 |