

Mitsunobu反应简便合成呋喃糖基苯并咪唑C-核苷
收稿日期: 2018-06-15
修回日期: 2018-08-21
网络出版日期: 2018-09-05
基金资助
国家自然科学基金(Nos.21372060,21772031)资助项目.
Concise Synthesis of Furanosyl Benzimidazole C-Nucleosides by Mitsunobu Reaction
Received date: 2018-06-15
Revised date: 2018-08-21
Online published: 2018-09-05
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372060, 21772031).
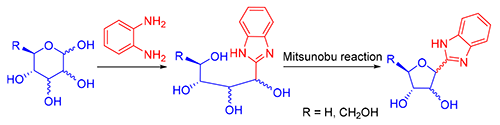
以不保护的五、六元单糖与邻苯二胺反应,得到中间体多羟基链苯并咪唑.进一步,利用Mitsunobu反应,分子内脱水合成了构型翻转和构型保持的呋喃糖基苯并咪唑C-核苷.Mitsunobu反应良好的区域选择性,为呋喃糖基苯并咪唑C-核苷的合成提供一个有效方法.
关键词: C-糖苷; 呋喃糖基苯并咪唑; Mitsunobu反应; 区域选择性
闫连海 , 侯宇恒 , 李小六 , 陈华 . Mitsunobu反应简便合成呋喃糖基苯并咪唑C-核苷[J]. 有机化学, 2018 , 38(12) : 3332 -3337 . DOI: 10.6023/cjoc201806025
Tetri/pentitolyl benzimidazoles were prepared by using the unprotected monosaccharides and o-phenylenediamine as the starting materials. Intramolecular dehydration of the oligotoltyl benzimidazoles afforded two furanosyl benzimidazole C-nucleosides (α/β isomers) through Mitsunoble reaction. One isomer was the configuration-retension product, the other was the configuration-inversion one. The regioselectivity of Mitsunobu reaction is good, which provides an effective protocol for the synthesis of furanosyl benzimidazole C-nucleosides.

[1] Yang, Y.; Yu, B. Chem. Rev. 2017, 117, 12281.
[2] Stambasky, J.; Hocek, M.; Kocovsky, P. Chem. Rev. 2009, 109, 6729.
[3] Bokor, E.; Kun, S.; Goyard, D.; Toth, M.; Praly, J.-P.; Vidal, S.; Somsak, L. Chem. Rev. 2017, 117, 1687.
[4] Lalitha, K.; Muthusamy, K.; Prasad, Y. S.; Vemula, P. K.; Nagarajan, S. Carbohydr. Res. 2015, 402, 158.
[5] Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Chem. Rev. 2018, 118, 1495.
[6] Zhang, Y. Z.; Chen, G. Y.; Chen, G. R. Chin. J. Org. Chem. 2008, 28, 321(in Chinese). (张云志, 陈冠宇, 陈国荣, 有机化学, 2008, 28, 321.)
[7] Akhtar, W.; Faraz Khan, M.; Verma, G.; Shaquiquzzaman, M.; Rizvib, M. A.; Hassan Mehdi, S.; Akhter, M.; Mumtaz Alam, M. Eur. J. Med. Chem. 2017, 126, 705.
[8] Bansal, Y.; Silakari, O. Bioorg. Med. Chem. Lett. 2012, 20, 6208.
[9] Cheng, Z.; Zhang, Q. F.; Xu, X. L.; Li, X. N. Chin. J. Org. Chem. 2015, 35, 1189(in Chinese). (程正, 张群峰, 许孝良, 李小年, 有机化学, 2015, 35, 1189.)
[10] Zhang, S.; Niu, Y. H.; Ye, X. S. Org. Lett. 2017, 19, 3608.
[11] Townsend, L. B.; Revankar, G. R. Chem. Rev. 1970, 70, 389.
[12] Sallam, M. A. E.; Abdel Hamid, O. G.; Gossen, V.; Raabe, G.; Aboulel, S. Carbohydr. Res. 2012, 361, 78.
[13] Lin, C.; Lai, P.-T.; Liao, S. K.-S.; Hung, W.-T.; Yang, W.-B.; Fang, J.-M. J. Org. Chem. 2008, 73, 3848.
[14] Qu, N.; Guo, J.; He, Y. Chin. J. Org. Chem. 2016, 36, 2880(in Chinese). (戚娜, 郭键, 贺耘, 有机化学, 2016, 36, 2880.)
[15] Wang, X. L.; Yang, F.; Xue, Z. Y.; Wang, X. Q. Chin. J. Org. Chem. 2015, 35, 29(in Chinese). (王小龙, 杨芳, 薛自燕, 王晓强, 有机化学, 2015, 35, 29.)
[16] Metlitskikh, S. V.; Koroteev, M. P.; Nifantyev, E. E. Russ. Chem. Bull., Int. Ed.. 2005, 54, 1272.
[17] Yadav, L. D. S.; Awasthi, C. Carbohydr. Res. 2010, 345, 318.
[18] Sallam, M. A. E. J. Chem. Soc., Perkin 11982, 557.
[19] CCDC-1849507 for 2a contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
[20] Zhang, W.; Li, M.; Ouyang, J.; Lin, W. H.; Deng, Z. W. J. Beijing Normal Univ. (Nat. Sci.) 2006, 42, 166(in Chinese). (张巍, 李敏, 欧阳捷, 林文瀚, 邓志威, 北京师范大学学报(自然科学版), 2006, 42, 166.)
[21] Hua, D. Y.; Yi, D. N.; Liu, J. N. Acta Pharm. Sin. 2003, 38, 946(in Chinese). (华丹宇, 易大年, 刘基宁, 药学学报, 2003, 38, 946.)
[22] Sallam, M. A. E.; Ibrahim, E. I.; El-Eter, K. A. A.; Cassady, J. M. Carbohydr. Res. 1997, 298, 93.
[23] Sallam, M. A. E.; El-Shemany, H. A. Carbohydr. Res. 1994, 261, 327.
[24] Moore, S.; Link, K. P. J. Org. Chem. 1940, 5, 637.
[25] Moore, S.; Link, K. P. J. Biol. Chem. 1940, 133, 293.
[26] Huebner, C. F.; Lohmap, R.; Dimiler, R. J.; Moore, S.; Link, K. P. J. Biol. Chem. 1943, 150, 503.
/
| 〈 |
|
〉 |