

多杀菌素A衍生物的合成及其生物活性研究
收稿日期: 2018-07-01
修回日期: 2018-08-02
网络出版日期: 2018-09-05
基金资助
国家自然科学基金(No.41471198)资助项目.
Study on the Synthesis and Insecticidal Activity of Spinosyn A Derivatives
Received date: 2018-07-01
Revised date: 2018-08-02
Online published: 2018-09-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 41471198).
张凯 , 李加荣 , 温都苏 , 刘洪林 , 王海邮 , 阿拉木斯 . 多杀菌素A衍生物的合成及其生物活性研究[J]. 有机化学, 2018 , 38(12) : 3363 -3372 . DOI: 10.6023/cjoc201807001
Spinosyns are a novel kind of biopesticide produced by Saccharopolyspora spinosa. Spinosyns have been widely applied in pest control because of their broad insecticidal spectrum, high insecticidal activity, low environmental impact, and low toxicity. To identify spinosyn compounds with improved insecticidal activity, a series of D-forosamine replacement derivatives of spinosyn A were synthesized. The insecticidal activities of the synthetic derivatives were evaluated against 3rd-instar larvae of Plutella xylostella. All derivatives exhibited good insecticidal activity to P. xylostella.
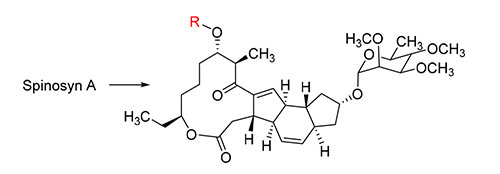
[1] Deng, X. L.; Zhang, L.; Hu, X. P.; Yin, B.; Liang P.; Yang, X. L. Chin. Chem. Lett. 2016, 27, 251.
[2] Liu, Y.; Lei, C.; Xu, X. Y.; Shao, X. S.; Li, Z. Chin. Chem. Lett. 2016, 27, 321.
[3] Lahm, G. P.; Cordova, D.; Barry, J. D. Med. Chem. 2009, 17, 4127.
[4] Wei, R. B.; Liu, Y.; Liang, Y. Chin. J. Org. Chem. 2009, 29, 476(in Chinese). (魏荣宝, 刘洋, 梁娅, 有机化学, 2009, 29, 476.)
[5] Mertz, F. P.; Yao, R. C. Int. J. Syst. Bacteriol. 1990, 40, 34.
[6] Legocki, J.; Polec, I.; Zelechowski, K. Pestycydy 2010, 33, 59.
[7] Thompson, G. D.; Dutton, R.; Sparks, T. C. Pest. Manage. Sci. 2000, 56, 696.
[8] Tillman, P. G.; Mulrooney, J. E. J. Econ. Entomol. 2000, 93, 1638.
[9] Boeck, L. D.; Chio, E. H.; Eaton, T. E.; Godfrey, O. W.; Michel, K. H.; Nakatsukasa, W. M.; Yao, R. C. EP 0375316, 1990.
[10] Baker, P. J. US 5227295, 1993.
[11] Turner, J. R.; HuberM, L. B.; Broughton, M. C.; Mynderse, J. S.; Martin, J. W. US 5591606, 1997.
[12] Mynderse, J. S.; Baker, P. J.; Mabe, J. A.; Broughton, M. C.; Huber, M. B.; Martin, J. W.; Turner, J. R. WO 9420518, 1994.
[13] Bacci, L.; Lupi, D.; Savoldelli, S.; Rossaro, B. J. Entomol. Acarolog. Res. 20016, 48, 40.
[14] Salgado, V. L.; Sheets, J. J.; Watson, G. B.; Schmidt, A. L. Pestic. Biochem. Physiol. 1998, 60, 103.
[15] Carson, W.; Trumble, J. Arthropod Manage. Tests 1998, 23, 74.
[16] Kerns, D. Arthropod Manage. Tests 1996, 21, 117.
[17] Linduska, J. Arthropod Manage. Tests 1998, 23, 95.
[18] Thompson, G. D.; Sparks, T. C. Synthesis and Chemistry of Agrochemicals, American Chemical Society, Washington, DC, 2002, Chapter 5, pp. 61~73.
[19] Cleveland, C. B.; Mayes, M. A.; Cryer, S. A. Pest Manage. Sci. 2002, 58, 70.
[20] Chai, H. X.; Shi, D. X.; Zhang Q.; Li, J. R. Chem. Ind. Eng. Prog. 2011, 30, 239(in Chinese). (柴洪新, 史大昕, 张奇, 李加荣, 化工进展, 2011, 30, 239.)
[21] Sparks, T. C.; Dripps, J. E.; Watson, G. B.; Paroonagian, D. Pestic. Biochem. Physiol. 2012, 102, 1.
[22] Hong, L.; Zhao, Z. B.; Melançon, C. E.; Zhang, H.; Liu, H. W. J. Am. Chem. Soc. 2008, 130, 4954.
[23] Erb, A.; Weiss, H.; Härle, J.; Bechthold, A. Phytochemistry 2009, 70, 1812.
[24] Fisher, M. H. Phytochemicals for Pest Control, American Chemical Society, Washington, DC, 1997, Chapter 17, pp. 220~238.
[25] Sheehan, L. S.; Lill, S. R. E.; Wilkinson, B.; Sheridan, R. M.; Vousden, W. A.; Kaja, A. L.; Crouse, G. D.; Gifford, J.; Graupner, P. R.; Karr, L.; Lewer, P.; Sparks, T. C.; Leadlay, P. F.; Waldron, C.; Martin, C. J. J. Nat. Prod. 2006, 69, 1702.
[26] Creemer, L. C.; Kirst, H. A.; Paschal, J. W. J. Antibiot. 1998, 51, 795.
[27] Daeuble, J.; Sparks, T. C.; Johnson, P.; Graupner, P. R. Bioorg. Med. Chem. 2009, 17, 4197.
[28] Tietze, L. F.; Brasche, G.; Grube, A.; Böhnke, N.; Stadler, C. Chem.-Eur. J. 2007, 13, 8543.
[29] Oliver, M. P.; Crouse, G. D.; Demeter, D. A.; Sparks, T. C. J. Agric. Food Chem. 2015, 63, 5571.
[30] Shi, D. X.; Feng, X.; Zhuang, X. L.; Chai, H. X.; Liu, A. J.; Liu, T.; Zhang, Q.; Li, J. R. Chin. J. Org. Chem. 2014, 34, 2543(in Chinese). (史大昕, 冯雪, 庄晓磊, 柴洪新, 柳安军, 刘霆, 张奇, 李加荣, 有机化学, 2014, 34, 2543.)
[31] Calabrese, A. WO 2017040763, 2017.
[32] Cheng, L. X. Mod. Agrochem. 2004, 3, 7(in Chinese). (程隆新, 现代农药, 2004, 3, 7.)
[33] Thomas C. Pestic. Biochem. Physiol. 2000, 67, 187.
[34] Creemer L. C.; Kirst H. A.; Paschal J. W. J. Antibiot. 1998, 51, 795.
[35] Tian, X. L.; Yin, X. H.; Long, Y. H.; Li, M.; Cai, T.; Li, R. Y.; Zhu, L. H. Chin. J. Pestic Sci. 2016, 18, 589(in Chinese). (田雪莲, 尹显慧, 龙友华, 李明, 蔡滔, 李荣玉, 朱流红, 农药学学报, 2016, 18, 589.)
[36] Wu, Y. L.; Wu, W. L.; Yao, Z. J.; Li, Y. L.; Wang, Y. F.; Li, J. C.; Sun, X. L.; Peng, Z. H.; Yu, Q.; Hu, T. S.; Zeng, B. B.; Wang, X. Z. Chin. J. Org. Chem. 2001, 21, 1051(in Chinese). (吴毓林, 吴文连, 姚祝军, 黎运龙, 王燕芳, 李金翠, 孙小玲, 彭陟辉, 俞千, 胡泰山, 曾步兵, 王祥柱, 有机化学, 2001, 21, 1051.)
[37] Hua, N. Z. Agrochemicals 2015, 54, 1(in Chinese). (华乃震, 农药, 2015, 54, 1.)
[38] Zhang, K.; Liu, S. L.; Liu, A. J.; Chai, H. X.; Li, J. R.; A, L. M. S. Beilstein J. Org. Chem. 20017, 13, 2603.
[39] Bai, Y.; Shen, X.; Li, Y.; Dai, M. J. Am. Chem. Soc. 2016, 138, 10838.
/
| 〈 |
|
〉 |