

镍催化下一溴二氟甲烷对(杂)芳基溴代物的二氟甲基化反应
收稿日期: 2018-08-15
修回日期: 2018-09-14
网络出版日期: 2018-10-12
基金资助
国家重点基础研究发展计划(973计划,No.2015CB931900)、国家自然科学基金(Nos.21425208,21672238,21332010,21421002)和中国科学院先导专项(No.XDB20000000)资助项目.
Nickel-Catalyzed Difluoromethylation of (Hetero)aryl Bromides with BrCF2H
Received date: 2018-08-15
Revised date: 2018-09-14
Online published: 2018-10-12
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2015CB931900), the National Natural Science Foundation of China (Nos. 21425208, 21672238, 21332010, 21421002), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000).
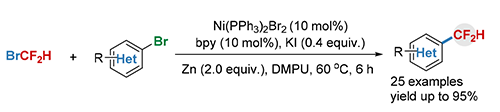
二氟甲基取代的(杂)芳烃化合物由于二氟甲基的独特性质越来越受到了化学家和药物学家的广泛关注.在过去的几年中,大量合成该类化合物的方法不断被发展,但这些方法中大多使用了价格昂贵的需要多步合成的二氟甲基化试剂,从而制约了其广泛应用.因此,发展以廉价易得的二氟甲基化试剂为氟源制备二氟甲基(杂)芳烃化合物的方法是十分必要的.以廉价易得的溴二氟甲烷(BrCF2H)为二氟甲基化试剂,发展了镍催化下(杂)芳基溴代物与BrCF2H的偶联反应.该反应高效温和,底物普适性好,官能团兼容性优秀,并且可以克量级合成,为合成二氟甲基(杂)芳烃化合物提供了一种高效简洁、廉价的方法.初步机理研究表明,该反应经历了一种镍催化的还原偶联历程.
高兴 , 何旭 , 张新刚 . 镍催化下一溴二氟甲烷对(杂)芳基溴代物的二氟甲基化反应[J]. 有机化学, 2019 , 39(1) : 215 -222 . DOI: 10.6023/cjoc201808014
A nickel-catalyzed direct difluoromethylation of (hetero)aryl bromides with bromodifluoromethane (BrCF2H) is described. This reaction features high efficiency, broad substrate scope and high functional group tolerance, providing a cost-efficient and straightforward route for applications in medicinal chemistry. Preliminary mechanistic studies reveal that a nickel-based, reductive cross-coupling catalytic cycle is involved in the reaction.

[1] (a) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529.
(b) Erickson, J. A.; McLoughlin, J. I. J. Org. Chem. 1995, 60, 1626.
[2] (a) Ge, S.; Chaladaj, W.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 4149.
(b) Gu, Y.; Leng, X.; Shen, Q. Nat. Commun. 2014, 5, 5405.
(c) Xu, L.; Vicic, D. A. J. Am. Chem. Soc. 2016, 138, 2536.
(d) Serizawa, H.; Ishii, K.; Aikawa, K.; Mikami, K. Org. Lett. 2016, 18, 3686.
[3] (a) Moore, G. G. I. J. Org. Chem. 1979, 44, 1708.
(b) Ruppert, I.; Schlich, K.; Volbach, W. Tetrahedron Lett. 1984, 25, 2195.
(c) Tyutyunov, A. A.; Boyko, V. E.; Igoumnov, S. M. Fluorine Notes 2011, 74, 1.
(d) Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Org. Chem. 2003, 68, 4457.
[4] Feng, Z.; Min, Q.-Q.; Zhang, X. Org. Lett. 2016, 18, 44.
[5] Feng, Z.; Min, Q.-Q.; Fu, X.-P.; An, L.; Zhang, X. Nat. Chem. 2017, 9, 918.
[6] Xu, C.; Guo, W.-H.; He, X.; Guo, Y.-L.; Zhang, X.-Y.; Zhang, X. Nat. Commun. 2018, 9, 1170.
[7] Guiadeen, D.; Kothandaraman, S.; Yang, L.; Mills, S. G.; MacCoss, M. Terahedron Lett. 2008, 49, 6368.
[8] Fu, X.-P.; Xiao, Y.-L.; Zhang, X. Chin. J. Chem. 2018, 36, 143.
[9] Sheng, J.; Ni, H.-Q.; Bian, K.-J.; Li, Y.; Wang, Y.-N.; Wang, X.-S. Org. Chem. Front. 2018, 5, 606.
[10] (a) Feng, Z.; Xiao, Y.-L.; Zhang, X. Acc. Chem. Res. 2018, 51, 2264.
(b) Feng, Z.; Chen, F.; Zhang, X. Org. Lett. 2012, 14, 1938.
(c) Feng, Z.; Min, Q.-Q.; Xiao, Y.-L.; Zhang, B.; Zhang, X. Angew. Chem., Int. Ed. 2014, 53, 1669.
(d) Min, Q.-Q.; Yin, Z.; Feng, Z.; Guo, W.-H.; Zhang, X. J. Am. Chem. Soc. 2014, 136, 1230.
(e) Xiao, Y.-L.; Guo, W.-H.; He, G.-Z.; Pan, Q.; Zhang, X. Angew. Chem., Int. Ed. 2014, 53, 9909.
[11] Prinsell, M. R.; Everson, D. A.; Weix, D. J. Chem. Commun. 2010, 46, 5743.
[12] 19F NMR showed the chemical shifts of difluoromethyl Zinc species A1 and A2 are consistent with the literature, see:Burton, D. J.; Hartgraves, G. A. J. Fluorine Chem. 2007, 128, 1198.
[13] (a) Weix, D. Acc. Chem. Res. 2015, 48, 1767.
(b) Gu, J.; Wang, X.; Xue, W.; Gong, H. Org. Chem. Front. 2015, 3, 1411.
[14] Lu, C.-H,; Gu, Y.; Wu, J.; Gu, Y.-C.; Shen, Q.-L. Chem. Sci. 2017, 8, 4848.
/
| 〈 |
|
〉 |