

基于芳基(三氟乙基)三价碘盐的三氟乙基化反应研究进展
收稿日期: 2018-08-25
修回日期: 2018-09-19
网络出版日期: 2018-10-20
基金资助
国家自然科学基金(No.21602165)、湖北省“楚天学者”计划、湖北省“百人计划”资助项目.
Progress on Trifluoroethylation Reactions Using Aryl(trifluoroethyl)iodonium Salts
Received date: 2018-08-25
Revised date: 2018-09-19
Online published: 2018-10-20
Supported by
Project supported by the National Natural Science Foundation of China (21602165), the "Chutian Scholar" Program from Department of Education of Hubei Province, and the "Hundred Talent" Program of Hubei Province.
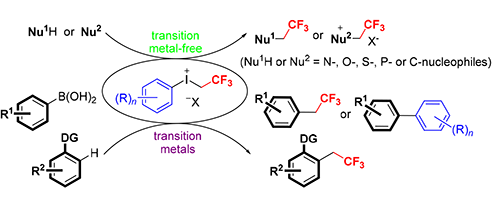
综述了过渡金属催化和无过渡金属催化的、以芳基(三氟乙基)三价碘盐为三氟乙基化试剂的三氟乙基化反应的研究进展.在这些反应中,大量不同类型的N-,O-,S-以及C-亲核性底物在温和的条件下被三氟乙基化.相比于其他三氟乙基化试剂,芳基(三氟乙基)三价碘盐具有更高的亲电反应活性.特别值得一提的是,双(三氟甲磺酰)亚胺芳基(三氟乙基)三价碘盐微溶于水、在水中稳定存在,并且可以在水溶液中对氨基酸衍生物和肽类化合物进行直接的三氟乙基化反应.将芳基(三氟乙基)三价碘盐用于芳烃的三氟乙基化反应时,它能够很好地克服反应使用其他试剂时带来的不足.这些研究成果也证明了过渡金属催化芳基(三氟乙基)三价碘盐进行直接的三氟乙基化反应的可能性.
关键词: 三氟乙基化; 无过渡金属催化; 过渡金属催化; 亲电; 芳基(三氟乙基)三价碘盐
韩秋燕 , 赵成龙 , 张成潘 . 基于芳基(三氟乙基)三价碘盐的三氟乙基化反应研究进展[J]. 有机化学, 2019 , 39(1) : 84 -94 . DOI: 10.6023/cjoc201808029
Transition metal-free and metal-catalyzed reactions using aryl(trifluoroethyl)iodonium salts as the trifluoroethylation reagents are summarized in this review. A large number of different types of N-, O-, S-, and C-nucleophiles were readily trifluoroethylated in these reactions under mild conditions. The results revealed that aryl(trifluoroethyl)iodonium salts possess much higher electrophilic reactivity than the other CH2CF3 sources. Especially, aryl(trifluoroethyl)iodonium bis(trifluoromethanesulfonyl)amides are stable and slightly soluble in water, which were successfully applied in the aqueous trifluoroethylation of amino acid derivatives and peptides. The utilization of aryl(trifluoroethyl)iodonium salts for aromatic trifluoroethylation has promisingly solved the problems that arise from the other reagents. These achievements have also demonstrated the synthetic possibilities of direct trifluoroethylation using aryl(trifluoroethyl)iodonium salts under transition-metal catalysis.

[1] (a) Kirsch, P. Modern Fluoroorganic Chemistry:Synthesis, Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, 2013.
(b) Gagnon, M.-C.; Auger, M.; Paquin, J.-F. Org. Biomol. Chem. 2018, 16, 4925.
(c) Nosova, E. V.; Lipunova, G. N.; Charushin, V. N.; Chupakhin, O. N. J. Fluorine Chem. 2018, 212, 51.
(d) Zhu, Y.; Han, J.; Wang, J.; Shibata, N.; Sodeoka, M.; Soloshonok, V. A.; Coelho, J. A. S.; Toste, F. D. Chem. Rev. 2018, 118, 3887.
(e) Meanwell, N. A. J. Med. Chem. 2018, 61, 5822.
(f) Wang, B.-C.; Wang, L.-J.; Jiang, B.; Wang, S.-Y.; Wu, N.; Li, X.-Q.; Shi, D.-Y. Mini-Rev. Med. Chem. 2017, 17, 683.
[2] (a) Uneyama, K.; Yamazaki, T. J. Fluorine Chem. 2017, 203, 3.
(b) Yang, L.; Dong, T.; Revankar, H.-M.; Zhang, C.-P. Green Chem. 2017, 19, 3951.
(c) Zhang, P.; Lv, L.; Shen, Q. Acta Chim. Sinica 2017, 75, 744(in Chinese). (张盼盼, 吕龙, 沈其龙, 化学学报, 2017, 75, 744.)
(d) Eisenstein, O.; Milani, J.; Perutz, R. N. Chem. Rev. 2017, 117, 8710.
(e) Song, H. X.; Han, Q. Y.; Zhao, C. L.; Zhang, C.-P. Green Chem. 2018, 20, 1662.
(f) Yang, J.; Zhao, H.-W.; He, J.; Zhang, C.-P. Catalysts 2018, 8, 23.
(g) Barata-Vallejo, S.; Cooke, M. V.; Postigo, A. ACS Catal. 2018, 8, 7287.
[3] (a) Han, J.-B.; Hao, J.-H.; Zhang, C.-P.; Qin, H.-L. Curr. Org. Chem. 2015, 19, 1554 and the references cited therein.
(b) Li, L.; Huang, M.; Liu, C.; Xiao, J.-C.; Chen, Q.-Y.; Guo, Y.; Zhao, Z.-G. Org. Lett. 2015, 17, 4714.
(c) Tang, X.-J.; Thomoson, C. S.; Dolbier, Jr. W. R. Org. Lett. 2014, 16, 4594.
(d) Han, E.-J.; Sun, Y.; Shen, Q.; Chen, Q.-Y.; Guo, Y.; Huang, Y.-G. Org. Chem. Front. 2015, 2, 1379.
(e) Luo, H.; Wu, G.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2015, 54, 14503.
(f) Yu, X.; Cohen, S. M. J. Am. Chem. Soc. 2016, 138, 12320.
(g) Mai, W.-P.; Sun, B.; Qian, G.-S.; Yuan, J.-W.; Mao, P.; Yang, L.-R.; Xiao, Y.-M. Tetrahedron 2015, 71, 8416.
(h) Roh, G.-b.; Iqbal, N.; Cho, E. J. Chin. J. Chem. 2016, 34, 459.
(i) Zhang, Y.; Du, H.; Zhu, M.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Tetrahedron Lett. 2017, 58, 880.
(j) Zheng, J.; Chen, Q.-Y.; Sun, K.; Huang, Y.; Guo, Y. Tetrahedron Lett. 2016, 57, 5757.
(k) Andrews, K. G.; Faizova, R.; Denton, R. M. Nat. Commun. 2017, 8, 15913.
[4] (a) Umemoto, T.; Gotoh, Y. J. Fluorine Chem. 1985, 28, 235.
(b) Umemoto, T.; Gotoh, Y. Bull. Chem. Soc. Jpn. 1987, 60, 3307.
(c) Lyalin, V. V.; Orda, V. V.; Alekseeva, L. A.; Yagupol'skii, L. M. J. Org. Chem. USSR 1972, 8, 1027.
[5] (a) Zhdankin, V. V.; Kuehl, C. K.; Simonsen, A. J. Tetrahedron Lett. 1995, 36, 2203.
(b) Zhdankin, V. V.; Kuehl, C. K.; Simonsen, A. J. J. Org. Chem. 1996, 61, 8272.
[6] Montanari, V.; Resnati, G. Tetrahedron Lett. 1994, 35, 8015.
[7] DesMarteau, D. D.; Montanari, V. Chem. Commun. 1998, 2241.
[8] Umemoto, T; Gotoh, Y. J. Fluorine Chem. 1986, 31, 231.
[9] Umemoto, T.; Gotoh, Y. Bull. Chem. Soc. Jpn. 1991, 64, 2008
[10] Tolnai, G. L.; Nilsson, U. J.; Olofsson, B. Angew. Chem., Int. Ed. 2016, 55, 11226.
[11] Han, Q.-Y.; Zhao, C.-L.; Yang, J.; Zhang, C.-P. Green Chem. Lett. Rev. 2017, 10, 162.
[12] Umemoto, T; Gotoh, Y. Bull. Chem. Soc. Jpn. 1987, 60, 3823.
[13] Zhao, C.-L.; Yang, J.; Han, Z.-Z.; Zhang, C.-P. J. Fluorine Chem. 2017, 204, 23.
[14] Tolnai, G. L.; Székely, A.; Makó, Z.; Gáti, T.; Daru, J.; Bihari, T.; Stirling, A.; Novák, Z. Chem. Commun. 2105, 51, 4488.
[15] DesMarteau, D. D.; Montanari, V. Chem. Lett. 2000, 29, 1052.
[16] (a) DesMarteau, D. D.; Lu, C. Tetrahedron Lett. 2006, 47, 561.
(b) DesMarteau, D. D.; Lu, C. J. Fluorine Chem. 2007, 128, 1326.
[17] (a) Lu, C.; DesMarteau, D. D. J. Fluorine Chem. 2007, 128, 832.
(b) Lu, C.; DesMarteau, D. D. Chem. Commun. 2008, 208.
[18] (a) Zhang, J.; Martin, G. R.; DesMarteau, D. D. Chem. Commun. 2003, 2334.
(b) Lu, C.; VanDerveer, D.; DesMarteau, D. D. Org. Lett. 2008, 10, 5565.
[19] Chu, A-H. A.; Minciunescu, A.; Montanari, V.; Kumar, K.; Bennett, S. C. Org. Lett. 2014, 16, 1780.
[20] Chu, A-H. A.; Minciunescu, A.; Bennett, S. C. Org. Lett. 2015, 17, 6262.
[21] (a) Yan, S.-Y.; Zhang, Z.-Z.; Shi, B.-F. Chem. Commun. 2017, 53, 10287.
(b) Zhang, H.; Wang, H.-Y.; Luo, Y.; Chen, C.; Cao, Y.; Chen, P.; Guo, Y.-L.; Lan, Y.; Liu, G. ACS Catal. 2018, 8, 2173.
(c) Zhang, X.; Yang, C. Adv. Synth. Catal. 2015, 357, 2721.
(d) Ohtsuka, Y.; Yamakawa, T. J. Fluorine Chem. 2016, 185, 96.
(e) Zhu, M.; Han, X.; Fu, W.; Wang, Z.; Ji, B.; Hao, X.-Q.; Song, M.-P.; Xu, C. J. Org. Chem. 2016, 81, 7282.
[22] Yang, J.; Han, Q.-Y.; Zhao, C.-L.; Dong, T.; Hou, Z.-Y.; Qin, H.-L.; Zhang, C.-P. Org. Biomol. Chem. 2016, 14, 7654.
[23] Tóth, B. L.; Kovács, S.; Sályi, G.; Novák, Z. Angew. Chem., Int. Ed. 2016, 55, 1988.
[24] Kovács, S.; Tóth, B. L.; Borsik, G.; Borsik, G.; Bihari, T.; May, N. V.; Stirling, A.; Novák, Z. Adv. Synth. Catal. 2017, 359, 527.
[25] Borah, A. J.; Shi, Z. Chem. Commun. 2017, 53, 3945.
[26] Maraswami, M.; Pankajakshan, S.; Chen, G.; Loh, T. P. Org. Lett. 2017, 19, 4223.
[27] Wen, D.; Yuan, B.; He, R.; Shen, W.; Li, M. Tetrahedron Lett. 2018, 59, 462.
/
| 〈 |
|
〉 |