

铜催化的芳基炔酮的羟化三氟甲硫基化反应
收稿日期: 2018-08-30
修回日期: 2018-10-23
网络出版日期: 2018-11-12
基金资助
国家自然科学基金(Nos.21332010,21421002)、中国科学院战略性先导科技专项(No.XDB20000000)和中国科学院青年创新促进会(No.2016234)资助项目.
Copper-Catalyzed Hydroxytrifluoromethylthiolation of Arylpropynones
Received date: 2018-08-30
Revised date: 2018-10-23
Online published: 2018-11-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21332010, 21421002), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (No. 2016234).
胡娟娟 , 黄焰根 , 徐修华 , 卿凤翎 . 铜催化的芳基炔酮的羟化三氟甲硫基化反应[J]. 有机化学, 2019 , 39(1) : 177 -182 . DOI: 10.6023/cjoc201808041
Recently, the preparation of fluorinated compounds through difunctionalization strategies has become a hot research area in fluorine chemistry. In this work, a copper-catalyzed hydroxytrifluoromethylthiolation of arylpropynones for the synthesis of the corresponding trifluoromethylthiolated enols was developed. The copper salt and solvent are crucial to the yields of this reaction. Under optimized reaction conditions, a series of trifluoromethylthiolated enols were obtained in moderate to good yields.
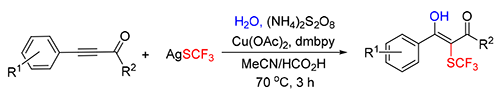
Key words: copper; trifluoromethylthiolation; hydroxylation; propynone; radical
[1] (a) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(b) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529.
(c) Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. Chem. Soc. Rev. 2012, 41, 31.
(d) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
(e) Gouverneur, V.; Seppelt, K. Chem. Rev. 2015, 115, 563.
(f) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422.
(g) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
(h) Meanwell, N. A. J. Med. Chem. 2018, 61, 5822.
[2] (a) Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207.
(b) Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
[3] (a) Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513.
(b) Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415.
(c) Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227.
(d) Xu, H.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731.
(e) Zhang, K.; Xu, X.-H.; Qing, F.-L. Chin. J. Org. Chem. 2015, 35, 556(in Chinese). (张珂, 徐修华, 卿凤翎, 有机化学, 2015, 35, 556.)
(f) Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445.
(g) Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150.
(h) Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397.
[4] (a) Sheng, J.; Li, S.; Wu, J. Chem. Commun. 2014, 50, 578.
(b) Zhang, K.; Liu, J.-B.; Qing, F.-L. Chem. Commun. 2014, 50, 14157.
(c) Zhu, L.; Wang, G.; Guo, Q.; Xu, Z.; Zhang, D.; Wang, R. Org. Lett. 2014, 16, 5390.
(d) Yang, T.; Lu, L.; Shen, Q. Chem. Commun. 2015, 51, 5479.
(e) Chen, D.-Q.; Gao, P.; Zhou, P.-X.; Song, X.-R.; Qiu, Y.-F.; Liu, X.-Y.; Liang, Y. M. Chem. Commun. 2015, 51, 6637.
(f) Fuentes, N.; Kong, W.; Fernández-Sánchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964.
(g) Luo, J.; Zhu, Z.; Liu, Y.; Zhao, X. Org. Lett. 2015, 17, 3620.
(h) Xu, C.; Shen, Q. Org. Lett. 2015, 17, 4561.
(i) Liu, X.; An, R.; Zhang, X.; Luo, J.; Zhao, X. Angew. Chem., Int. Ed. 2016, 55, 5846.
(j) Zhang, P.; Li, M.; Xue, X.-S.; Xu, C.; Zhao, Q.; Liu, Y.; Wang, H.; Guo, Y.; Lu, L.; Shen, Q. J. Org. Chem. 2016, 81, 7486.
(k) Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486.
(l) Luo, J.; Liu, Y.; Zhao, X. Org. Lett. 2017, 19, 3434.
(m) Pan, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 4624.
(n) Liu, X.; Liang, Y.; Ji, J.; Luo, J.; Zhao, X. J. Am. Chem. Soc. 2018, 140, 4782.
(o) Xiao, Z.; Liu, Y.; Zheng, L.; Liu, C.; Guo, Y.; Chen, Q.-Y. J. Org. Chem. 2018, 83, 5836.
(p) Xi, C.-C.; Chen, Z.-M.; Zhang, S.-Y.; Tu, Y.-Q. Org. Lett. 2018, 20, 4227.
[5] Wu, W.; Dai, W.; Ji, X.; Cao, S. Org. Lett. 2016, 18, 2918.
[6] (a) Guo, C.-H.; Chen, D.-Q.; Chen, S.; Liu, X.-Y. Adv. Synth. Catal. 2017, 359, 2901.
(b) Li, H.; Liu, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Chem. Commun. 2017, 53, 10136.
[7] (a) Tlili, A.; Alazet, S.; Glenadel, Q.; Billard, T. Chem.-Eur. J. 2016, 22, 10230.
(b) Pan, S.; Li, H.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 3247.
[8] Jiang, L.; Ding, T.; Yi, W.-B.; Zeng, X.; Zhang, W. Org. Lett. 2018, 20, 2236.
[9] (a) Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem., Int. Ed. 2009, 48, 8551.
(b) Wu, J.-J.; Xu, J.; Zhao, X. Chem.-Eur. J. 2016, 22, 15265.
[10] (a) Yang, Y.; Jiang, X.-L.; Qing, F.-L. J. Org. Chem. 2012, 77, 7538.
(b) Wu, X.; Chu, L.; Qing, F.-L. Angew. Chem. Int. Ed. 2013, 52, 2198.
(c) Jiang, X.-Y.; Qing, F.-L. Angew. Chem. Int. Ed. 2013, 52, 14177.
(d) Yu, W.; Xu, X.-H.; Qing, F.-L. Adv. Synth. Catal. 2015, 357, 2039.
(e) Yang, B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2015, 17, 1906.
(f) Lin, Q.-Y.; Xu, X.-H.; Zhang, K.; Qing, F.-L. Angew. Chem., Int. Ed. 2016, 55, 1479.
(g) Yang, B.; Xu, X.-H.; Qing, F.-L. Chin. J. Chem. 2016, 34, 465.
(h) Lin, Q.-Y.; Ran, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 2419.
(i) Yu, W.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 5130.
(j) Yang, B.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 5956.
(k) Yang, B.; Yu, D.; Xu, X.-H.; Qing, F.-L. ACS Catal. 2018, 8, 2839.
(l) Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2018, 57, 6926.
[11] (a) Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128.
(b) Guo, S.; Zhang, X.; Tang, P. Angew. Chem., Int. Ed. 2015, 54, 4065.
(c) Wu, H.; Xiao, Z.; Wu, J.; Guo, Y.; Xiao, J.-C.; Liu, C.; Chen, Q.-Y. Angew. Chem., Int. Ed. 2015, 54, 4070.
(d) Qiu, Y.-F.; Zhu, X.-Y.; Li, Y.-X.; He, Y.-T.; Yang, F.; Wang, J.; Hua, H.-L.; Zheng, L.; Wang, L.-C.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2015, 17, 3694.
(e) Zeng, Y.-F.; Tan, D.-H.; Chen, Y.; Lv, W.-X.; Liu, X.-G.; Li, Q.; Wang, H. Org. Chem. Front. 2015, 2, 1511.
(f) Li, C.; Zhang, K.; Xu, X.-H.; Qing, F.-L. Tetrahedron Lett. 2015, 56, 6273.
(g) Pan, S.; Huang, Y.; Qing, F. L. Chem. Asian J. 2016, 11, 2854.
(h) Huang, F.-Q.; Wang, Y.-W.; Sun, J.-G.; Xie, J.; Qi, L.-W.; Zhang, B. RSC Adv. 2016, 6, 52710.
(i) Song, Y.-K.; Qian, P.-C.; Chen, F.; Deng, C.-L.; Zhang, X.-G. Tetrahedron 2016, 72, 7589.
(j) Ji, M. S.; Wu, Z.; Yu, J. J.; Wan, X. B.; Zhu, C. Adv. Synth. Catal. 2017, 359, 1959.
(k) He, B.; Xiao, Z.; Wu, H.; Guo, Y.; Chen, Q.-Y.; Liu, C. RSC Adv. 2017, 7, 880.
(l) Liu, K.; Jin, Q.; Chen, S.; Liu, P. N. RSC Adv. 2017, 7, 1546.
(m) Li, M.; Petersen, J. L.; Hoover, J. M. Org. Lett. 2017, 19, 638.
(n) Cheng, Z.-F.; Tao, T.-T.; Feng, Y.-S.; Tang, W.-K.; Xu, J.; Dai, J.-J.; Xu, H.-J. J. Org. Chem. 2018, 83, 499.
[12] (a) Ye, Y.; Sanford, M. S. J. Am. Chem. Soc. 2012, 134, 9034.
(b) Huang, L.; Zheng, S.-C.; Tan, B.; Liu, X.-Y. Chem.-Eur. J. 2015, 18, 6718.
(c) Yu, L.-Z.; Wei, Y.; Shi, M. Chem. Commun. 2016, 52, 13163.
(d) Cheng, C.; Liu, S.; Lu, D.; Zhu, G. Org. Lett. 2016, 18, 2852.
[13] (a) Wu, W.; Zhang, X.; Liang, F.; Cao, S. Org. Biomol. Chem. 2015, 13, 6992.
(b) Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem. Int. Ed. 2018, 57, 12690.
/
| 〈 |
|
〉 |