

新型哌啶噻唑类化合物的合成及杀虫活性
收稿日期: 2018-09-05
修回日期: 2018-10-22
网络出版日期: 2018-11-26
基金资助
浙江省绿色农药2011协同创新中心开放基金资助项目.
Synthesis and Insecticidal Activity of Novel Piperidine Thiazole Compounds
Received date: 2018-09-05
Revised date: 2018-10-22
Online published: 2018-11-26
Supported by
Project supported by the Collaborative Innovation Center of Zhejiang Province Green Pesticide.
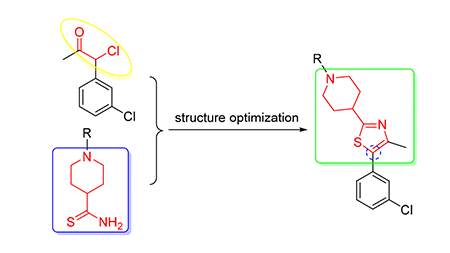
为了探寻新型的生物活性化合物,设计并合成了12个未见文献报道的新型哌啶噻唑类衍生物.生物活性测试研究发现,在500 μg/mL浓度下,目标化合物对粘虫表现出良好的抑制活性,而且在100 μg/mL下,(4-(5-(3-氯苯基)-4-甲基噻唑-2-基)哌啶-1-基)(4-甲基哌嗪-1-基)甲酮(1f)、(5-(3-氯苯基)-4-甲基噻唑-2-基)哌啶-1-基)(4-硝基-1H-吡唑-3-基)甲酮(1g)对粘虫的抑制率均达80%以上,另外在20 μg/mL下(5-(3-氯苯基)-4-甲基噻唑-2-基)哌啶-1-基)(4-硝基-1H-吡唑-3-基)甲酮(1g)仍具有50%的杀虫活性.
丁成荣 , 潘亚运 , 殷许 , 谭成侠 , 张国富 . 新型哌啶噻唑类化合物的合成及杀虫活性[J]. 有机化学, 2019 , 39(3) : 836 -841 . DOI: 10.6023/cjoc201809009
Twelve new piperdine thiazole compounds were designed and synthesized in search of new bioactive compounds. The preliminary bioassay showed that at the concentration of 500 μg/mL the lethal rates of the target compounds possessed certain insceticidal activities against armyworm, and at the concentration of 100 μg/mL the lethal rates of (4-(5-(3-chloro-phenyl) -4-methylthiazol-2-yl) piperidin-1-yl) (4-methylpiperazin-1-yl) methanone (1f) and (4-(5-(4-chlorophenyl) -4-methyl-thiazol-2-yl) piperidin-1-yl) nitro-1H-pyrazol-3-yl) methanone (1g) against armyworm were 80% and 100%, respectively. Further screening at concentrations of 20 μg/mL, the lethal rate of (4-(5-(4-chlorophenyl) -4-methylthiazol-2-yl) piperidin-1-yl) (4-nitro-1H-pyrazol-3-yl) methanone (1g) against armyworm was 50%.

[1] Huang, G.; Yang, J. -C.; Li, H.-C. Agrochemicals 2011, 50, 79(in Chinese.) (黄光, 杨吉春, 李慧超, 农药, 2011, 50, 79.)
[2] Yang, Z.-H.; Tian, H.; Zhang, L. World Pestic. 2017, 39, 43(in Chinese). (杨子辉, 田昊, 张莉, 世界农药, 2017, 39, 43.)
[3] Tan, Y.; Zhang, B.-H. J. Math. Med. 2012, 25, 89(in Chinese). (覃宇, 张宝徽, 数理医药学杂志, 2012, 25, 89.)
[4] Biller, S. A. US 5739135, 1998[Chem. Abstr. 1998, 128, 282780].
[5] Guedat, P. US 20070054939, 2007[Chem. Abstr. 2007, 142, 93809].
[6] Li, Y. CN 106588911, 2017[Chem. Abstr. 2017, 166, 515724].
[7] Fan, Z. J. CN 104650060, 2015[Chem. Abstr. 2015, 163, 65937].
[8] Gu, L.-L.; Bai, Y.-L. Mod. Agrochem. 2017, 16, 42(in Chinese.) (顾林玲, 柏亚罗, 现代农药, 2017, 16, 42.)
[9] He, X.-L. World Pestic. 2015, 37, 57(in Chinese). (何秀玲, 世界农药, 2015, 37, 57.)
[10] Cederbaum, F. WO 2014118142, 2014[Chem. Abstr. 2014, 161, 320959].
[11] Lamberth, C. WO 2014154530, 2014[Chem. Abstr. 2014, 161, 640429].
[12] Tomoki, T. US 20130296272, 2013[Chem. Abstr. 2012, 156, 337332].
[13] Gregory, V. WO 2009055514, 2009[Chem. Abstr. 2009, 150, 578441].
[14] Stefan, H. US 20160198713, 2016[Chem. Abstr. 2016, 162, 400022].
[15] Pierre, C. US 20110312999, 2011[Chem. Abstr. 2011, 155, 615369].
[16] Hanagan, M. A. WO 2009094407, 2009[Chem. Abstr. 2009, 151, 234956].
[17] Kamireddy, B. WO 2009094445, 2009[Chem. Abstr. 2009, 151, 173451].
[18] Pasteris, R. J. WO 2008013925, 2008[Chem. Abstr. 2008, 148, 185163].
[19] Yi, A.-Q.; Xue, S.-J.; Fang, Z.-K. Chin. J. Org. Chem. 2009, 29, 454(in Chinese). (尹安琴, 薛思佳, 方治坤, 有机化学, 2009, 29, 454.)
[20] Hong, Y.; Dai, H.; Ye, Y.-L. Chin. J. Org. Chem. 2017, 37, 3006(in Chinese). (洪宇, 戴红, 叶林玉, 有机化学, 2017, 37, 3006.)
[21] Gao, H.; Zheng, X.; Zhu, P. Chin. J. Org. Chem. 2018, 38, 684(in Chinese). (高慧, 郑喜, 朱萍, 有机化学, 2018, 38, 684.)
[22] Zhang, Z.-H.; Chen, Y.; Cai, B.-S. Chin. J. Org. Chem. 2017, 37, 2377(in Chinese). (张志华, 陈羽, 柴宝山, 有机化学, 2017, 37, 2377.)
[23] Cao, L.; Sun, J.-W.; Liu, Q. Chin. J. Org. Chem. 2017, 37, 3031(in Chinese). (曹蕾, 孙景伟, 刘强, 有机化学, 2017, 37, 3031.)
[24] Ma, H.-L.; Yan, X.-J.; Xiao, Y.-M. Chin. J. Org. Chem. 2016, 36, 158(in Chinese). (麻红利, 闫晓静, 肖玉梅, 有机化学, 2016, 36, 158.)
[25] Sun, N.; Wang, X.; Ding, Z.-B. Chin. J. Org. Chem. 2016, 36, 2489(in Chinese). (孙楠, 王欣, 丁志彬, 有机化学, 2016, 36, 2489.)
[26] Li, R.; Nakashima, T.; Galangau, O. Chem. Asian J. 2015, 10, 1725.
[27] Khillare, L. D.; Pratap, U. R.; Bhosle, M. R. Chem. Intermed. 2017, 12, 1.
[28] Tong, D.; Duan, H.; Wang, J. Chem. Res. Chin. Univ. 2014, 30, 4.
[29] Hantzsch, A.; Weber, J. H. Chem. Ber. 1887, 20, 3118.
[30] Parsons P. J.; Johnathan. B.; Waters A. J. Synth. Commun. 2007, 37, 985.
[31] Srivatsavati, Jagapathi, R.; Pothukuchi, S.; Rani, S.-S. WO 2012143933, 2012[Chem. Abstr. 2012, 167, 481902].
[32] Dai, H.; Ye, L. Y.; Zhuang, H. Y.; Dai, B. J.; Fang, Y.; Shi, Y. J. Molecules 2015, 20, 21870.
/
| 〈 |
|
〉 |