

砂贝母中异甾体生物碱类成分的研究
收稿日期: 2018-09-12
修回日期: 2018-10-19
网络出版日期: 2018-11-26
基金资助
国家自然科学基金新疆联合基金(No.U1403201)资助项目.
Isosteroidal Alkaloids from Fritillaria karelinii
Received date: 2018-09-12
Revised date: 2018-10-19
Online published: 2018-11-26
Supported by
Project supported by the National Natural Science Foundation of China and the People's Government of Xinjiang Uygur Autonomous Region (NSFC-Xinjiang Joint Fund, No. U1403201).
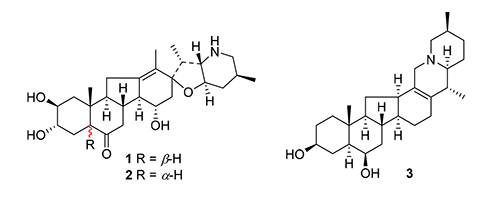
利用各种柱色谱和高效液相色谱等分离纯化方法,从砂贝母鳞茎中分离得到6个异甾体生物碱类化合物.根据质谱、一维/二维核磁共振谱和X射线单晶衍射等技术鉴定了它们的结构,分别为karelinine(1),5-epikarelinine(2),27-epiebeienine(3),ebeienine(4),persicanidine B(5)和heilonine(6),其中化合物1~3为新化合物.化合物1是贝母属中罕见的5β-jervine型异甾体生物碱,具有A/B环顺式稠合方式.化合物1和2也是贝母属中首次发现的具有15α-OH取代的jervine型生物碱.
关键词: 砂贝母; 异甾体生物碱; karelinine; 5-epikarelinine; 27-epiebeienine
黄金昌, 雷春, 阿吉艾克拜尔·艾萨, 俞媚华, 阿布力米提·伊力, 侯爱君 . 砂贝母中异甾体生物碱类成分的研究[J]. 有机化学, 2019 , 39(3) : 842 -847 . DOI: 10.6023/cjoc201809019
Three new isosteroidal alkaloids, karelinine (1), 5-epikarelinine (2), and 27-epiebeienine (3), were isolated from the bulbs of Fritillaria karelinii by column chromatography and semipreparative HPLC (high performance liquid chro-matography), together with three known ones, ebeienine (4), persicanidine B (5), and heilonine (6). Their structures were identified by MS, 1D/2D NMR, and single-crystal X-ray diffraction. Compound 1 is a 5β-jervine isosteroidal alkaloid featuring a cis-fused A/B ring moiety, rarely found in the Fritillaria genus. Compounds 1 and 2 also represent the first jervine alkaloids with a 15 α-hydroxy group from this genus.

[1] Nanjing University of Chinese Medicine Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Press, Shanghai, 2005, pp. 296~299, 1274~1275, 3383~3384(in Chinese). (南京中医药大学, 中药大辞典, 上海科学技术出版社, 上海, 2005, pp. 296~299, 1274~1275, 3383~3384.)
[2] Chinese Materia Medica Editorial Committee of State Administration of Traditional Chinese Medicine Chinese Materia Medica, Vol. 22, Shanghai Science and Technology Press, Shanghai, 1999, pp. 88~101(in Chinese). (国家中医药管理局《中华本草》编委会, 中华本草, 第22卷, 上海科学技术出版社, 上海, 1999, pp. 88~101.)
[3] Li, Y.; Yili, A.; Li, J.; Muhamat, A.; Aisa, H. A. Bioorg. Med. Chem. Lett. 2016, 26, 1983.
[4] Wang, D. D.; Wang, S.; Chen, X.; Xu, X. L.; Zhu, J. Y.; Nie, L. H.; Long, X. J. Ethnopharmacol. 2012, 139, 189.
[5] Li, H. J.; Jiang, Y.; Li, P. Nat. Prod. Rep. 2006, 23, 735.
[6] Hao, D. C.; Gu, X. J.; Xiao, P. G.; Peng, Y. Chin. J. Nat. Med. 2013, 11, 330.
[7] Shen, S.; Li, G.; Huang, J.; Chen, C.; Ren, B.; Lu, G.; Tan, Y.; Zhang, J.; Li, X.; Wang, J. Fitoterapia 2012, 83, 785.
[8] Shou, Q. Y.; Tan, Q.; Shen, Z. W. Tetrahedron Lett. 2009, 50, 4185.
[9] Pi, H. F.; Zhang, P.; Ruan, H. L.; Zhang, Y. H.; Sun, H. D.; Wu, J. Z. J. Asian Nat. Prod. Res. 2009, 11, 779.
[10] Lin, B. Q.; Ji, H.; Li, P.; Jiang, Y.; Fang, W. Eur. J. Pharmacol. 2006, 551, 125.
[11] Chen, X. Q.; Mordak, H. V. Flora of China, Vol. 24, Science Press, Beijing, 2000, p. 132.
[12] Pan, X. F.; Zhu, Z. Q. J. Lanzhou Univ. 1979, 24, 54(in Chinese). (潘鑫复, 朱子清, 兰州大学学报, 1979, 24, 54.)
[13] Liu, Y. M.; Feng, Y. D.; Lu, X.; Nie, J. B.; Li, W.; Wang, L. N.; Tian, L. J.; Liu, Q. H. Eur. J. Med. Chem. 2017, 137, 280.
[14] Richmond, V.; Garrido Santos, G. A.; Murray, A. P.; Maier, M. S. Steroids 2011, 76, 1160.
[15] Wu, J. Z.; Pan, X. P.; Lou, M. A.; Wang, X. S.; Ling, D. K. Acta Pharm. Sin. 1989, 24, 600(in Chinese). (吴继洲, 潘锡平, 娄民安, 王孝生, 凌大奎, 药学学报, 1989, 24, 600.)
[16] Lee, P.; Kitamura, Y.; Kaneko, K.; Shiro, M.; Xu, G. J.; Chen, Y. P.; Hsu, H. Y. Chem. Pharm. Bull. 1988, 36, 4316.
[17] Ori, K.; Mimaki, Y.; Sashida, Y.; Nikaido, T.; Ohmoto, T. Phytochemistry 1992, 31, 3605.
[18] Kitamura, Y.; Nishizawa, M.; Kaneko, K. Tetrahedron 1989, 45, 7281.
[19] Li, Q.; Yang, K. X.; Zhao, Y. L.; Qin, X. J.; Yang, X. W.; Liu, L.; Liu, Y. P.; Luo, X. D. J. Ethnopharmacol. 2016, 179, 274.
[20] Cong, Y.; Li, J.; Zhang, Q. C.; Guo, J. G.; Li, S. S. Helv. Chim. Acta 2015, 98, 85.
[21] Zhou, X. F.; Gao, Z. G.; Han, X. R.; Zhao, W. J.; Wang, S. S. Chin. Chem. Lett. 2010, 21, 1209.
[22] Du, Y.; Zheng, Z. G.; Yu, Y.; Wu, Z. T.; Liang, D.; Li, P.; Jiang, Y.; Li, H. J. J. Pharm. Biomed. Anal. 2017, 142, 201.
[23] Atta-ur-Rahman; Farooq, A.; Choudhary, M. I.; Gilani, A. H.; Shaheen, F.; Ali, R. A.; Noor-e-ain, F.; Sener, B. Planta Med. 1994, 60, 377.
/
| 〈 |
|
〉 |