

金属催化剂催化醇和胺直接偶联制备亚胺的研究进展
收稿日期: 2018-08-30
修回日期: 2018-11-14
网络出版日期: 2018-12-05
基金资助
山东省高等教育科技计划(No.J16LC13)资助项目.
Progress in Imine Formation from Direct Coupling of Alcohols and Amines Catalyzed by Metal Catalysts
Received date: 2018-08-30
Revised date: 2018-11-14
Online published: 2018-12-05
Supported by
Project supported by the Higher Educational Science and Technology Program of Shandong Province (No. J16LC13).
王辉 , 黄龙江 . 金属催化剂催化醇和胺直接偶联制备亚胺的研究进展[J]. 有机化学, 2019 , 39(4) : 883 -902 . DOI: 10.6023/cjoc201808039
Imines are very important class of compounds and have been widely utilized in fine chemicals, pharmaceuticals and chemical industry. The C=N double bond in imine is an important nitrogen source in different types of reactions due to its high reactive activity. Due to its high atom economy, catalytic direct coupling of alcohols and amines to imines based on metal catalysts has attracted much attention and maken great progress in recent years. In this paper, the advances in direct coupling of alcohols and amines to imines catalyzed by metal catalysts are reviewed.
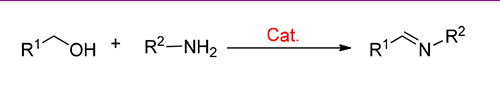
Key words: imine; alocohols; amines; metal catalysis
[1] Marques, C. S.; Burke, A. J. ChemInform 2011, 3, 635.
[2] Bayrak, H.; Demirbas, A.; Karaoglu, S. A.; Demirbas, N. Eur. J. Med. Chem. 2009, 44, 1057.
[3] Gawronski, J.; Wascinska, N.; Gajewy, J. Chem. Rev. 2008, 108, 5227.
[4] Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626.
[5] And, S. K.; Ishitani, H. Chem. Rev. 1999, 99, 1069.
[6] Nielsen, M.; Worgull, D.; Zweifel, T.; Gschwend, B.; Bertelsen, S.; Jørgensen, K. A. Chem. Commun. 2011, 47, 632.
[7] Marques, C. S.; Burke, A. J. ChemCatChem 2011, 3, 635.
[8] Uematsu, N.; Fujii, A.; Hashiguchi, S.; Ikariya, T.; Noyori, R. J. Am. Chem. Soc. 1996, 118, 4916.
[9] Thalji, R. K.; Ahrendt, K. A.; Bergman, R. G.; Ellman, J. A. ChemInform 2010, 33, 9692.
[10] Nieto, S.; Dragna, J. M.; Anslyn, E. V. Chem.-Eur. J. 2010, 16, 227.
[11] Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. ChemCatChem 2010, 2, 1438.
[12] Nakajima, R.; Ogino, T.; Yokoshima, S.; Fukuyama, T. J. Am. Chem. Soc. 2010, 132, 1236.
[13] Hadjipavlou-Litina, D. J.; Geronikaki, A. A. Drug Des. Discovery 1998, 15, 199.
[14] Akhmetova, V. R.; Khabibullina, G. R.; Rakhimova, E. B. Mol. Diversity 2010, 14, 463.
[15] Nielsen, M.; Worgull, D.; Zweifel, T.; Gschwend, B.; Bertelsen, Jørgensen, K. A. Chem. Commun. 2011, 47, 632.
[16] Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626.
[17] Xie, J. H.; Zhu, S. F.; Zhou, Q. L. Chem. Rev. 2011, 111, 1713.
[18] Marques, C. S.; Burke, A. J. ChemCatChem. 2011, 3, 635.
[19] Nieto, S.; Dragna, J. M.; Anslyn, E. V. Chem.-Eur. J. 2010, 16, 227.
[20] Dhakshinamoorthy, A.; Alvaro, M. ChemCatChem 2010, 2, 1438.
[21] Aschwanden, L.; Mallat, T.; Maciejewski, M.; Krumeich, F.; Baiker, A. ChemCatChem 2010, 2, 666.
[22] Min, S. K.; Kim, S.; Park, S.; Park, S.; Bosco, W.; Chidrala, R. K.; Park, J. J. Org. Chem. 2009, 74, 2877.
[23] Jiang, L.; Jin, L. L.; Tian, H. W.; Yuan, X. Q.; Yu, X. C.; Xu, Q. Chem. Commun. 2011, 47, 10833.
[24] Chen, G. J.; Ma, H. C.; Xin, W. L.; Li, X. B.; Jin, F. Z.; Wang, J. S.; Liu, M. Y.; Dong, Y. B. Inorg. Chem. 2017, 56, 654.
[25] Tian, H. W.; Yu, X. C.; Li, Q.; Wang, J. X.; Xu, Q. Adv. Synth. Catal. 2012, 354, 2671.
[26] Kang, Q.; Zhang, Y. G. Green Chem. 2012, 43, 1016.
[27] Bai, L.; Dang, Z. RSC Adv. 2015, 5, 10341.
[28] Darapanani, C. M.; Arghya, S.; Galia, M. J. Catal. 2017, 355, 139.
[29] Wei, Y. G.; Yu, H.; Zhai, Y. Y.; Dai, G. Y.; Ru, S.; Han, S. Chem.-Eur. J. 2017, 23, 13883.
[30] And, L. B.; Taylor R, J. K. Org. Lett. 2001, 4, 1637.
[31] Sithambaram, S.; Kumar, R.; Son, Y. C.; Steven, L. J. Catal. 2008, 253, 269.
[32] Mondal, J.; Borah, P.; Sreejith, S.; Nguyen, K. T.; Han, X. G.; Ma, X.; Zhao, Y. L. ChemCatChem 2014, 6, 3518.
[33] Chen, B.; Li, J.; Dai, W.; Wang, L. Y.; Gao, S. Green Chem. 2014, 16, 3328.
[34] Zhang, E, L.; Tian, H. W.; Xu, S. D.; Yu, X. C.; Xu, Q. Org. Lett. 2013, 15, 2704.
[35] Geng, L. L.; Song, J. L.; Zheng, B.; Wu, S. J.; Zhang, W. X.; Jia, M. J.; Liu, G. Chin. J. Catal. 2016, 37, 1451.
[36] Sindhuja, E.; Ramesh, R. Tetrahedron Lett. 2014, 55, 5504.
[37] Sun, H.; Su, F. Z.; Ni, J.; Cao, Y.; He, H. Y.; Fan, K. N. Angew. Chem., Int. Ed. 2009, 48, 4390.
[38] Kegnæs, S.; Mielby, J.; Mentzel, U. V.; Christensen, C. H.; Riisager, A. Green Chem. 2010, 12, 1437.
[39] Liu, P.; Li, C.; Hensen, E. J. Chem.-Eur. J. 2012, 18, 12122.
[40] Soulé, J. F.; Miyamura, H.; Kobayashi, S. Chem. Commun. 2013, 49, 355.
[41] Huang, R.; Yang, Y.; Wang, D. S.; Zhang, L.; Wang, D. W. Org. Chem. Front. 2017, 5, 203.
[42] Han, L.; Xing, P.; Jiang, B. Org. Lett. 2014, 16, 3428.
[43] Mielby, J.; Poreddy, R.; Engelbrekt, C.; Kegnæs, S. Chin. J. Catal. 2014, 35, 670.
[44] Zhang, G. Q.; Hanson, S. K. Org. Lett. 2013, 15, 650.
[45] Sun, Y. W.; Lu, X. H.; Wei, X. L.; Zhou, D.; Xia, Q. H. Catal. Commun. 2014, 43, 213.
[46] Midya, S. P.; Pitchaimani, J.; Landge, V. G.; Madhu, V.; Ekam-baram, B. Catal. Sci. Technol. 2018, 8, 3469.
[47] Xu, C.; Lai, Goh, L. Y.; Pullarkat, S. A. Organometallics. 2011, 30, 6499.
[48] Chang, Y. H.; Tanigawa, I.; Takeuchi, K.; Taguchi, H.; Ozawa, F. Eur. J. Inorg. Chem. 2016, 5, 754.
[49] Gnanaprakasam, B.; Zhang, J.; Milstein, D. Angew. Chem., Int. Ed. 2010, 49, 1468.
[50] Cano, R.; Ramón, D. J.; Yus, M. J. Org. Chem. 2011, 76, 5547.
[51] Maggi, A.; Madsen, R. Organometallics 2012, 31, 451.
[52] Jared, W. R.; Sara, A. M.; Simon, D. P.; Jennifer, M. S. Org. Biomol. Chem. 2012, 10, 1746.
[53] Musa, S.; Fronton, S.; Vaccaro, L.; Gelman, D. Organometallics 2013, 32, 3069.
[54] Saha, B.; Daw, P.; Sengupta, G.; Rahaman, S. M.; Bera, J. K. Chem.-Eur. J. 2014, 20, 6542.
[55] Oldenhuis, N. J.; Dong, V. M.; Guan. Z. B. Tetrahedron 2014, 70, 4213.
[56] Higuchi, T.; Tagawa, R.; Iimuro, A.; Akiyama, S.; Nagae, H.; Mashima, K. Chem.-Eur. J. 2017, 23, 12795.
[57] Yu, X. J.; Li, Y. Q.; Fu, H. Y.; Zheng, X. L.; Chen, H.; Li, R. X. Appl. Organomet. Chem. 2018, 32, 4277.
[58] Shiraishi, Y.; Ikeda, M.; Tsukamoto, D.; Tanaka, S.; Hirai, T. Chem. Commun. 2011, 47, 4811.
[59] Pérez, J. M.; Cano, R.; Yus, M.; Ramón, D. J. Eur. J. Org. Chem. 2012, 24, 4548.
[60] Tang, L.; Sun, H. Y.; Li, Y. F.; Zha, Z. G.; Wang, Z. Y. Green Chem. 2012, 14, 3423.
[61] Wang, H.; Zhang, J.; Cui, Y. M.; Yang, K. F.; Zheng, Z. J.; Xu, L. W. RSC. Adv. 2014, 4, 34681.
[62] Bain, J.; Cho, P.; Voutchkova-Kostal, A. Green Chem. 2015, 17, 2271.
[63] Jaiswal, G.; Landge, V. G.; Jagadeesan, D.; Balaraman, E. Green Chem. 2016, 47, 3232.
[64] Mukherjee, A.; Nerush, A.; Leitus, G.; Shimon L, J. W.; David, Y. B.; Jalapa N, A. E.; Milstein, D. J. Am. Chem. Soc. 2016, 138, 4298.
[65] Mastalir, M.; Glatz, M.; Gorgas, N.; Stçger, B.; Pittenauer, E.; Allmaier, G.; Veiros, L. F.; Kirchner, K. Chem.-Eur. J. 2016, 22, 12316.
[66] Fertig, R.; Irrgang, T.; Freitag, F.; Zander, J.; Kempe, R. ACS Catal. 2018, 9, 8525.
[67] Esteruelas, M. A.; Honczek, N.; Valencia, M.; Oñate, E.; Oliván, M. Organometallics 2011, 30, 2468.
[68] Madsen, R.; Azizi, K. ChemCatChem 2018, 53, 1.
/
| 〈 |
|
〉 |