

新型席夫碱Cr3+荧光探针及其细胞成像研究
收稿日期: 2018-09-21
修回日期: 2018-10-29
网络出版日期: 2018-12-07
基金资助
国家自然科学基金(No.U1404207)资助项目.
A Novel Schiff-Base Fluorescent Probe for Cr3+ and Its Bioimaging in Cells
Received date: 2018-09-21
Revised date: 2018-10-29
Online published: 2018-12-07
Supported by
Project supported by the National Natural Science Foundation of China (No.U1404207).
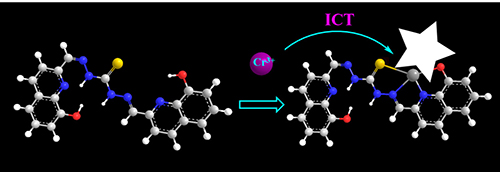
Cr3+离子在脂肪、核酸、糖和蛋白质的代谢中发挥着重要作用,并且Cr3+离子被认为是一种致癌物质,对人类有极大的危害.因此,通过将8-羟基喹啉醛和硫代碳酰肼反应制备出化合物L.探针L的CH3CN/H2O (V:V=1:2,Tris buffer 50 mmol/L,pH=7.3)溶液中加入Cr3+离子,可以通过肉眼观察到其颜色从无色到黄色的变化和明显的荧光增强效应,这些现象表现出探针L对Cr3+离子具有良好的选择性.根据Job曲线、荧光滴定、质谱和密度泛函理论(DFT)计算确定了探针L与Cr3+离子形成1:1的络合物.络合常数为1.00×105 L/mol,检测限为2.85×10-7mol/L.此外,生物成像实验表明,探针L可以通过荧光增强信号检测活细胞中的Cr3+离子.
常永新 , 李白 , 高云帆 , 徐括喜 . 新型席夫碱Cr3+荧光探针及其细胞成像研究[J]. 有机化学, 2019 , 39(4) : 1023 -1028 . DOI: 10.6023/cjoc201809028
Trivalent chromium (Cr3+) plays an important role in the metabolismof fats, nucleic acids, carbohydrates and proteins. Moreover, Cr3+ is considered to be a carcinogen and is extremely harmful to humans. Therefore, Cr3+ fluorescent probe L was synthesized by reaction of 8-hydroxyquinolinaldehyde with thiocarbazone. The probe L showed high selectivity towards Cr3+ ion through the color of solution changed from colorless to yellow for naked-eye detection and significant fluorescence intensity enhanced in CH3CN/H2O (V:V=1:2, Tris buffer 50 mmol/L, pH=7.3) solution. The 1:1 binding stoichiometry between probe and Cr3+ was determined from Job's plot, fluorescence titration, ESI-MS and density functional theory (DFT) calculations. The association constant (Ka) and the detection limit for Cr3+ were found to be to be 1.00×105 and 2.85×10-7 mol/L, respectively. Moreover, bioimaging experiments showed that L could sense Cr3+ ion in living cells with a fluorescence enhancement signal.

Key words: Cr3+; fluorescent probe; DFT; bioimaging
[1] Vincent, J. B. Proc. Nutr. Soc. 2004, 63, 41.
[2] Filik, H.; Dondurmacioglu, F. J. Anal. Chem. 2009, 64, 455.
[3] Wang, Y. T. J. Ind. Microbiol. Biotechnol 1995, 14, 159.
[4] Filik, H.; Dondurmacioglu, F. J. Anal. Chem. 2009, 64, 455.
[5] Chalmardia, G. B.; Tajbakhsha, M. Tetrahedron 2018, 18, 40.
[6] Gou, C.; Qin, S. H.; Wang, Y.; Liu, X. Y. Inorg. Chem. Commun. 2011, 14, 1622.
[7] Azadbakht, R.; Keypour, H. Spectrochim. Acta, Part A 2012, 85, 293.
[8] Vincent, J, B. Nutr. Rev. 2000, 58, 67.
[9] Anderson, R. A. Regul. Toxicol. Pharmacol. 1997, 26, 35.
[10] Filik, H.; Dondurmacioglu, F. J. Anal. Chem. 2009, 64, 455.
[11] Gómez, V.; Callao, M. P. TrAC, Trends Anal. Chem. 2006, 25, 1006.
[12] Lu, J. S.; Tian, J. Y.; Wu, H. Chin. J. Anal. Chem. 2009, 37, 99.
[13] Xu, K. X.; Kong, H. J.; Li, Q.; Song, P.; Dai, Y. P.; Li, Y. Spectrochim. Acta, Part A 2015, 137, 957.
[14] Kamakura, N.; Inui, T.; Kitano, M.; Nakamura, T. Spectrochim. Acta, Part B 2014, 93, 28.
[15] Li, M.; Zhang, D.; Liu, Y.; Ding, P. J. Fluorescence 2014, 24, 119.
[16] Noorbasha, N. M.; Jiangb, S. J. Talanta 2009, 80, 173.
[17] Zhang, N.; Suleiman, J. S.; He, M. Talanta 2008, 75, 536.
[18] Aydan, F. A.; Soylak, M. J. Hazard. Mater. 2010, 173, 669.
[19] Xie, X.; Tang, F.; Shangguan, X.; Che, S.; Niu J.; Xiao, Y.; Wang, X.; Tang, B. Chem. Commun. 2017, 53, 6520.
[20] Dai, Y. P.; Fu, J. X.; Yao, K.; Song, Q. Q.; Xu, K. X. Spectrochim. Acta, Part A 2018, 192, 257.
[21] Dolaia, M.; Sahab, U.; Dasc, A. K.; Kumarb, G. S. Anal. Methods-UK 2018, 10, 4063.
[22] Liu, X. Y.; Wang, P.; Fu, J. X.; Yao, K.; Xue, K.; Xu, K. X. J. Lumin. 2017, 186, 16.
[23] Brewer, T. F.; Chang, C. J. J. Am. Chem. Soc. 2015, 137, 10886.
[24] Keck, J.; Kramer, H. E. A.; Port, H.; Hirsch, T.; Fischer, P.; Rytz, G. J. Phys. Chem. 1996, 100, 14468.
[25] Hou, S. H.; Qu, Z. G.; Zhong, K. H.; Bian, Y. J.; Tang, L. J. Chin. J. Org. Chem. 2016, 36, 768(in Chinese).(侯淑华, 曲忠国, 钟克利, 边延江, 汤立军, 有机化学, 2016, 36, 768.)
[26] Yao, K.; Fu, J. X.; Chang, Y. X.; Li, B.; Li, Y.; Xu, K. X. Spectrochim. Acta, Part A 2018, 205, 410.
[27] Zhang, P.; Wang, H.; Zhang, D.; Zeng, X.; Zeng, R.; Xiao, L.; Tao, H.; Long, Y.; Yi, P.; Chen, J. Sens. Actuators, B 2018, 255, 2223.
[28] Fu, Y. J.; Yao, H. W.; Zhu, X. Y.; Guo, X. F.; Wang, H. Anal. Chim. Acta 2017, 994, 1.
[29] Dai, Y. P.; Liu, X. Y.; Wang, P.; Fu, J. X.; Yao, K.; Xu, K. X. RSC Adv. 2016, 6, 99933.
[30] Wang, P.; Fu, J. X.; Yao, K. Chang, Y. X.; Xu, K. X. Sens. Actuators, B 2018, 273, 1070.
[31] Samir, M.; Milan, S.; Debasish, D.; Prativa, M.; Gobinda, P.; Sahoo, A. Sens. Actuators, B 2017, 248, 223.
/
| 〈 |
|
〉 |