

含噁二唑杨梅素衍生物的合成及生物活性研究
收稿日期: 2018-09-28
修回日期: 2018-11-02
网络出版日期: 2018-12-17
基金资助
国家重点研发计划(No.2017YFD0200506)、国家自然科学基金(No.21867003)资助项目.
Synthesis and Biological of Novel Myricetin Derivatives Containing 1,3,4-Oxadiazoles
Received date: 2018-09-28
Revised date: 2018-11-02
Online published: 2018-12-17
Supported by
Project supported by the National Key Research and Development Program of China (No.2017YFD0200506),and the National Natural Science Foundation of China (No.21867003).
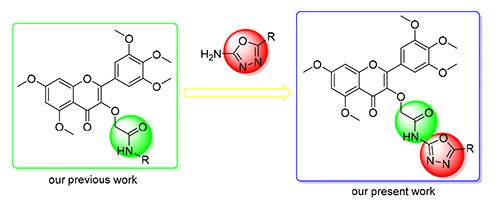
合成了一系列含有1,3,4-噁二唑的杨梅素衍生物,所有化合物经1H NMR,13C NMR以及HRMS表征.生物活性测试表明,部分化合物对柑橘溃疡病菌(Xac)、水稻白叶枯病菌(Xoo)以及烟草花叶病病毒(TMV)具有较好的抑制作用.其中化合物4a、4b、4f、4j对柑橘溃疡病菌的EC50分别为18.5、40.7、26.9和32.4 μg/mL,优于对照药叶枯唑(68.8 μg/mL)·化合物4f、4j对水稻白叶枯的EC50分别为45.9和35.7 μg/mL,优于对照药叶枯唑(69.3 μg/mL)·对TMV治疗活性,化合物4n的EC50值为272.8 μg/mL,优于对照药宁南霉素(428.8 μg/mL)·对TMV保护活性,化合物4f的EC50值为235.6 μg/mL,优于对照药宁南霉素(447.9 μg/mL).化合物4j与南方水稻黑条矮缩病毒P9-1作用的微量热涌动实验表明,该化合物与P9-1之间具有较强的相互作用.
张橙 , 蒋仕春 , 陈英 , 郭涛 , 夏榕娇 , 汤旭 , 陈丽娟 , 贺鸣 , 薛伟 . 含噁二唑杨梅素衍生物的合成及生物活性研究[J]. 有机化学, 2019 , 39(4) : 1160 -1168 . DOI: 10.6023/cjoc201809040
A series of novel myricetin derivatives containing 1,3,4-oxadiazole moiety were designed and synthesized.Bioassays indicated that some compounds showed potential antibacterial and antiviral activities. Among them, compounds 4a,4b, 4f and 4j demonstrated appreciable inhibitory effect against Xanthomonas axonopodis pv.citri (Xac), with half-maximal effective concentration (EC50) values of 18.5, 40.7, 26.9 and 32.4 μg/mL, which were significantly better than commercial agent bismerthiazol (68.8 μg/mL), compounds 4f and 4j also demonstrated appreciable inhibitory effect against Xanthomonas oryzae pv. Oryzae (Xoo) with EC50 values of 45.9 and 35.7 μg/mL, which were better than commercial agent bismerthiazol (69.3 μg/mL). In addition, compounds 4n demonstrated significant curative activity against TMV with EC50 value of 272.8 μg/mL, which was better than commercial agent ningnamycin (428.8 μg/mL), compounds 4f showed protecting activity against tobacco mosaic virus (TMV) with EC50 value of 235.6 μg/mL, which was better than commercial agent ningnamycin (447.9 μg/mL). Microscale thermophoresis (MST) indicated that compound 4j could bind with south rice black drawf virus P9-1.

Key words: myricetin; oxadiazole; biological activity; protein
[1] Bos, L. Trends Microbiol. 2000, 8, 82.
[2] Li, X. Y.; Liu, J.; Yang, X.; Ding, Y.; Wu, J.; Hu, D. Y.; Song, B. A. Bioorg. Med. Chem. Lett. 2015, 23, 3629.
[3] Zhou, G. H.; Wen, J. J.; Cai, D. J.; Li, P.; Xu, D. L.; Zhang, S. G. Chin. Sci. Bull. 2008, 53, 3677.
[4] Huang, N.; Angeles, E. R.; Domingo, J.; Magpanty, G.; Singh, S.; Zhan, G.; Kumarvadivel, N.; Bennett, J.; Khush, G. S. Theor. Appl. Genet. 1997, 95, 313.
[5] Guo, R.-X.; Li, L.-G.; Wang, Y.-F.; Huo, C.-H.; Fu, Y.; Wang, L.; Shi, W.-Q. Chin. Tradit. Herbal Drugs 2015, 46, 2019(in Chinese).(郭瑞霞, 李力更, 王于方, 霍长虹, 付炎, 王磊, 史文清, 中草药, 2015, 46, 2019.)
[6] Liu, C.-L.; Li, Z.-M. Pesticides 2003, 42, 1(in Chinese).(刘长令, 李正名, 农药, 2003, 42, 1.)
[7] Mei, Q.-G.; Yuan, W.-C.; Wang, C. Chin. J. Org. Chem. 2015, 35, 70(in Chinese).(梅青刚, 袁伟成, 王淳, 有机化学, 2015, 35, 70.)
[8] Yu, M. S.; Lee, J.; Lee, J. M.; Kim, Y.; Chin, Y. W.; Jee, J. G.; Keum, Y. S.; Jeong, Y. J. Bioorg. Med. Chem. Let. 2012, 22, 4049.
[9] Nguyen, T. H. V.; Trinh, A. V.; Nguyen, X. N.; Kiem, P. V.; Minh, C. V.; Long, P. Q.; Anh, L.T.; Cuong, N. M.; Song, J. H.; Ko, H. J.; Kim, N, Park, S. J.; Kim, S. H. Nat. Prod. Commun. 2014, 9, 643.
[10] Zhong, X. M.; Wang, X. B.; Chen, L. J.; Ruan, X. H.; Li, Q.; Zhang, J. P.; Chen, Z.; Xue, W. Chem. Cent. J. 2017, 11, 106.
[11] Chen, C. C; Huang, C. Y. Protein J. 2011, 30, 59.
[12] Rashed, K.; Ciric, A.; Glamoclija, J.; Sokovic, M. Ind. Crop. Prod. 2014, 59, 210.
[13] Xiao, W.; Ruan, X.-H.; Li, Q.; Zhang, J.-P.; Zhong, X.-M.; Xie, Y.; Wang, X.-B.; Huang, M.-G.; Xue, W. Chem. J. Chin. Univ. 2017, 38, 35(in Chinese).(肖维, 阮祥辉, 李琴, 张菊平, 钟新敏, 谢艳, 王晓斌, 黄民国, 薛伟, 高等学校化学学报, 2017, 38, 35.)
[14] Chobot, V.; Hadacek, F. Redox Rep. 2011, 16, 242.
[15] Zhao, L.; Xu, S. P.; Li, Z. Y.; Zhang, L.; Zhang, Z. S.; Pan, R. L. Sci. Technol. Food Ind. 2012, 33, 56.
[16] Xue, W.; Song, B. A.; Zhao, H. J. Eur. J. Med. Chem. 2015, 97, 155.
[17] Tae, K. H.; Inae, J.; Mi, E. K.; Bae, S. K.; Lee, J. K. Biomed. Pharmacother. 2017, 91, 378.
[18] Huang, M.-G.; Ruan, X.-H.; Zhang, J.-P.; Li, Q.; Wang, Y.-H.; Chen, L.-J.; Zhang, C.; Li, P. Chin. J. Org. Chem. 2017, 37, 2145(in Chinese).(黄民国, 阮祥辉, 张菊平, 李琴, 王一会, 陈丽娟, 张橙, 李普, 有机化学, 2017, 37, 2145.)
[19] Ningaiah, S.; Bhadraiah, U. K.; Doddaramappa, S. D.; Keshavamurthy, K. Bioorg. Med. Chem. Lett. 2014, 24, 245.
[20] Li, P.; Shi, L.; Gao, M. L.; Yang, X.; Xue, W.; Jin, L. H.; Hu, D. Y.; Song, B. A. Bioorg. Med. Chem. Lett. 2015, 25, 481.
[21] Gan, X. H.; Hu, D. Y.; Chen, Z.; Wang, Y. J.; Song, B. A. Bioorg. Med. Chem. Lett. 2017, 27, 4298.
[22] Wu, W. N; Chen, Q.; Tai, A. Q.; Jiang, G. Q.; Ouyang, G. P. Bioorg. Med. Chem. Lett. 2015, 25, 2243.
[23] Ragab, F. A. F.; Abou-Seri, S. M.; Abdel-Aziz, S. A.; Alfayomy, A. M; Aboelmagd, M. Eur. J. Med. Chem. 2017, 138, 140
[24] Zhang, S.; Luo, Y.; He, L. Q.; Liu, Z. J.; Jiang, A. Q.; Yang, Y. H.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2013, 21, 3723..
[25] Aziz-Ur-Rehman; Siddiqui, S. Z.; Abbasi, M. A.; Abbas, N.; Khan, K. M.; Shahid, M.; Mahmood, Y. Int. J. Pharm. Pharm. Sci. 2012, 4, 676.
[26] Rajak, H.; Kharya, M. D.; Mishra, P. Arch. Pharm. Chem. Life Sci. 2008, 341, 247.
[27] Niu, P. F.; Kang, J. F.; Tian, X. H.; Song, L. N.; Liu, H. X.;Wu, J.; Yu, W. Q.; Chang, J. B. J. Org. Chem. 2015, 80, 1018.
/
| 〈 |
|
〉 |