

非预期的重排反应及苯并噁唑类化合物的合成
收稿日期: 2018-09-28
修回日期: 2018-11-14
网络出版日期: 2019-01-10
基金资助
国家自然科学基金(Nos.21776060,21276064)、河北省自然科学基金(No.B2016205165)资助项目.
Unexpected Rearrangement Reaction and Synthesis of Benzoxazoles
Received date: 2018-09-28
Revised date: 2018-11-14
Online published: 2019-01-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos.21776060,21276064),and the Natural Science Foundation of Hebei Province (No.B2016205165).
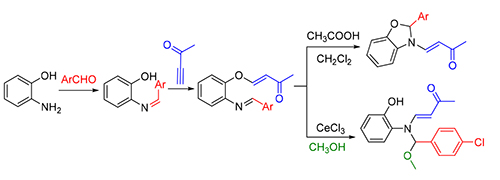
报道了一类酸促进的非预期的重排反应,合成了6个2-芳基-3-(3-氧代丁烯基)-苯并噁唑化合物6a~6f·提出了各步反应的反应机理,采用密度泛函理论(DFT)方法,从原子布居电荷和分子能量两方面对机理进行理论分析.研究结果表明,理论和实验一致说明本文中的反应机理的正确.重排反应机理的提出,为进一步研究该类反应提供了依据,为氮杂环类化合物的合成提供了方法参考.
关键词: 重排反应; 苯并噁唑衍生物; 合成; 反应机理; 密度泛函理论(DFT)
王凯璇 , 王兰芝 . 非预期的重排反应及苯并噁唑类化合物的合成[J]. 有机化学, 2019 , 39(4) : 1147 -1152 . DOI: 10.6023/cjoc201809038
Novel series of rearrangement reactions were herein reported that enable access to a variety of unique 2-aryl-3-(3'-oxobutenyl)-benzoxazole compounds 6a~6f from 2-aminophenol, aromatic aldehyde and 3-butyn-2-one as materials by nucleophilic conjugate addition, dehydration and rearrangement reactions and intramolecular cyclization in the presence of a catalytic amount of CH3COOH in CH2Cl2 at ambient temperature. On the basis of products and intermediate products, a series of possible mechanism was presented and theoretically verified by density functional theory (DFT) method at B3LYP/6-31G (d,p) level from both molecular energy and atomic charge in Gaussian 03 package. The results show that the theory and experiment consistently explain the rationality of the reaction mechanism. The mechanism of rearrangement provides a basis for further study of this type of reaction. The advantage of this method is that a novel structure of benzoxazole derivative was synthesized successfully via a series of rearrangement reactions. Therefore, this method can be used as an attractive strategy for practical synthesis of nitrogen heterocyclic compounds.

[1] Zhou, W. J.; Zhang, L.; Xiao, W.; Chen, H. J.; Wu, W. N.; Ouyang, G. P. J. Heterocycl. Chem. 2017, 54, 1423.
[2] Praveen, C.; Nandakumar, A.; Dheenkumar, P.; Muralidharan, D.; P. Perumal, P. T. J. Chem. Sci. 2012, 124, 609.
[3] Temiz-Arpaci, O.; Arisoy, M.; Sac, D.; Doganc, F.; Tasci, M.; Senol, F. S.; Orhan, I. E. Z. Naturforsch., C 2016, 71, 409.
[4] Reen, G. K,; Kumar, A.; Sharma, P. Med. Chem. Res. 2017, 26, 3336.
[5] Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Eur. J. Med. Chem. 2004, 9, 291.
[6] Tasci, M.; Temiz-Arpaci, O.; Kaynak-Onurdag, F.; Okten, S. Indian J. Chem., Sect. B:Org. Chem. Incl. Med. Chem. 2018, 57, 385.
[7] Tseng, C.-H.; Lin, C.-K.; Chen, Y.-L.; Tseng, C.-K.; Lee, J.-Y. Lee, J.-C. Eur. J. Med. Chem. 2018, 143, 970.
[8] Henderson, J. A.; Bilimoria, D.; Bubenik, M.; Cadilhac, C.; Cottrell, K. M.; Denis, F.; Dietrich, E.; Ewing, N.; Falardeau, G.; Giroux, S.; L'Heureux, L.; Liu, B.; Mani, N.; Morris, M.; Nicolas, O.; Pereira, O. Z.; Poisson, C.; Reddy, T. J.; Selliah, S.; Shawgo, R. S.; Vaillancourt, L.; Wang, J.; Xu, J.; Chauret, N.; Berlioz-Seux, F.; Chan, L. C.; Das, S. K.; Grillot, A.-L.; Bennani, Y. L.; Maxwell, J. P. Bioorg. Med. Chem. Lett. 2015, 25, 948.
[9] Zilifdar, F.; Foto, E.; Ertan-Bolelli, T.; Aki-Yalcin, E.; Yalcin, I.; Diril, N. Arch. Pharm. 2018, 351.
[10] Khajondetchairit, P.; Phuangsawai, O.; Suphakun, P.; Rattanabunyong, S.; Choowongkomon, K.; Gleeson, M. P. Chem. Biol. Drug Des. 2017, 90, 987.
[11] Goekhan-Kelekci, N.; Koeksal, M.; Uenuevar, S.; Aktay, G.; Erdogan, H. J. Enzyme Inhib. Med. Chem. 2009, 24, 29.
[12] Eren, G.; Unlu, S.; Nunez, M. T.; Labeaga, L.; Ledo, F.; Entrena, A.; Banoglu, E.; Costantino, G.; Sahin, M. F. Bioorg. Med. Chem. 2010, 18, 6367.
[13] Jayanna, N. D.; Vagdevi, H. M.; Dharshan, J. C.; Raghavendra, R. Telkar, S. B. Med. Chem. Res. 2013, 22, 5814.
[14] Wei, P.-F.; Qi, M.-Z.; Wang, Z.-P.; Ding, S.-Y.; Yu, W.; Liu, Q.; Wang, L.-K.; Wang, H.-Z.; An, W.-K.; Wang, W. J. Am. Chem. Soc. 2018, 140, 4623.
[15] Yeh, V. S. C. Tetrahedron 2004, 60, 11995.
[16] Leaver, I. H.; Milligan, B. Dyes Pigm. 1984, 5, 109.
[17] Chen, T.-R. J. Organomet. Chem. 2008, 693, 3117.
[18] Dunwell, D. W.; Evans, D. Hicks, T. A. J. Med. Chem. 1975, 18, 1158.
[19] Grossi, G.; Di Braccio, M.; Roma, G.; Ballabeni, V.; Tognolini, M.; Calcina, F.; Barocelli, E. Eur. J. Med. Chem. 2002, 37, 933
[20] Smith, R. H., Jr.; Jorgensen, W. L.; Tirado-Rives, J. Lamb, M. L.; Janssen, P. A.; Michejda, C. J.; Kroeger Smith, M. B. J. Med. Chem. 1998, 41, 5272
[21] Liu, D.; Chen, H. Y.; Zhang, J. Y.; Huang, J. Y.; Li, Y. M.; Peng, Q. M. Appl. Surf. Sci. 2018, 456, 59.
[22] Li, X.-Q. Li; Wang, L.-Z. Chin. Chem. Lett. 2014, 25, 327.
[23] Yin, L.-Y.; Wang, L.-Z. Tetrahedron Lett. 2016, 57, 5935.
[24] Qiu, Z.-L.; Wang, L.-Z.; Li, W.-H.; Li. Y. Acta Chim. Sinica 2011, 69, 1217(in Chinese).(邱召来, 王兰芝, 李文红, 李媛, 化学学报, 2011, 69, 1217.)
/
| 〈 |
|
〉 |