

基于咔唑和三嗪单元的位点调控设计并合成高效蓝光热活化延迟荧光分子
收稿日期: 2018-10-14
修回日期: 2018-12-05
网络出版日期: 2019-01-10
基金资助
国家自然科学基金(Nos.21572152,51873139)资助项目.
Design and Synthesis of Highly Efficient Blue Thermally Activated Delayed Fluorescence Molecules Based on Carbazole and 1,3,5-Triazine Units through Position Engineering
Received date: 2018-10-14
Revised date: 2018-12-05
Online published: 2019-01-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572152, 51873139).
王彤彤 , 华晓晨 , 郁友军 , 袁熠 , 冯敏强 , 蒋佐权 . 基于咔唑和三嗪单元的位点调控设计并合成高效蓝光热活化延迟荧光分子[J]. 有机化学, 2019 , 39(5) : 1436 -1443 . DOI: 10.6023/cjoc201810016
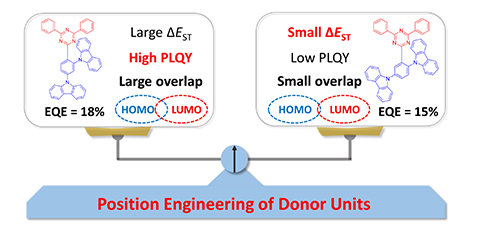
Using carbazole as the donor motif, triazine as the acceptor motif, two thermally activated delayed fluorescence (TADF) molecules of m-CzTri and p-CzTri were designed and synthesized through efficient combination and position regulation. Both materials emit bright blue fluorescence with very small singlet-triplet splitting energy. Computational simulations show that the donor and acceptor groups are well separated from the highest occupied orbit (HOMO) and the lowest unoccupied orbit (LUMO). In addition, their thermal, electrochemical, photophysical, and device properties were tested. Among them, the color coordinates of organic light emitting diode (OLED) devices with m-CzTri as the emitter were (0.15, 0.25), and the external quantum efficiency was as high as 18%.
[1] Tang, C. W.; Vanslyke, S. A. Appl. Phys. Lett. 1987, 51, 913.
[2] Adachi, C. Jpn. J. Appl. Phys. 2014, 53, 060101.
[3] Baldo, M. A.; O'Brien, D. F.; Thompson, M. E.; Forrest, S. R. Phys. Rev. B 1999, 60, 14422.
[4] Baldo, M. A.; O'Brien, D. F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M. E.; Forrest, S. R. Nature 1998, 395, 151.
[5] Wang, Y.-K.; Sun, Q.; Wu, S.-F.; Yuan, Y.; Li, Q.; Jiang, Z.-Q.; Fung, M.-K.; Liao, L.-S. Adv. Funct. Mater. 2016, 26, 7929.
[6] Yang, S.; Meng, F.; Wu, X.; Wu, X.; Yin, Z.; Liu, X.; You, C.; Wang, Y.; Su, S.; Zhu, W. J. Mater. Chem. C 2018, 6, 5769.
[7] Méhes, G.; Nomura, H.; Zhang, Q.; Nakagawa, T.; Adachi, C. Angew. Chem., Int. Ed. 2012, 51, 11311.
[8] Pan, K.-C.; Li, S.-W.; Ho, Y.-Y.; Shiu, Y.-J.; Tsai, W.-L.; Jiao, M.; Lee, W.-K.; Wu, C.-C.; Chung, C.-L.; Chatterjee, T.; Li, Y.-S.; Wong, K.-T.; Hu, H.-C.; Chen, C.-C.; Lee, M.-T. Adv. Funct. Mater. 2016, 26, 7560.
[9] Yuan, Y.; Hu, Y.; Zhang, Y.-X.; Lin, J.-D.; Wang, Y-K; Jiang, Z.-Q.; Liao L.-S. Adv. Funct. Mater. 2017, 27, 1700986.
[10] Liang, X.; Yan, Z.-P.; Han, H.-B.; Wu, Z.-G.; Zheng, Y.-X.; Meng, H.; Zuo, J.-L.; Huang, W. Angew. Chem. 2018, 130, 11486.
[11] Matsui, K.; Oda, S.; Yoshiura, K.; Nakajima, K.; Yasuda, N.; Hatakeyama, T. J. Am. Chem. Soc. 2018, 140, 1195.
[12] Xie, Y.; Li, Z. J. Polym. Sci. Part A: Polym. Chem. 2017, 55, 575.
[13] Goushi, K.; Yoshida, K.; Sato, K.; Adachi, C. Nat. Photonics 2012, 6, 253.
[14] Zhu, M. Z.; Yang, C. L. Chem. Soc. Rev. 2013, 42, 4963.
[15] Im, Y.; Kim, M.; Cho, Y. J.; Seo, J.-A.; Yook, K. S.; Lee, J. Y. Chem. Mater. 2017, 29, 1946.
[16] Einzinger, M.; Zhu, T.; De Silva, P.; Belger, C.; Swager, T. M.; Van Voorhis, T.; Baldo, M. A. Adv. Mater. 2017, 29, 1701987.
[17] Xie, G.; Li, X.; Chen, D.; Wang, Z.; Cai, X.; Chen, D.; Li, Y.; Liu, K.; Cao, Y.; Su, S. J. Adv. Mater. 2016, 28, 181.
[18] Liu, M.; Komatsu, R.; Cai, X.; Hotta, K.; Sato, S.; Liu, K.; Chen, D.; Kato, Y.; Sasabe, H.; Ohisa, S.; Suzuri, Y.; Yokoyama, D.; Su, S.-J.; Kido, J. Chem. Mater. 2017, 29, 8630.
[19] Etherington, M. K.; Gibson, J.; Higginbotham, H. F.; Penfold, T. J.; Monkman, A. P. Nat. Commun. 2016, 7, 13680.
[20] Shizu, K.; Noda, H.; Tanaka, H.; Taneda, M.; Uejima, M.; Sato, T.; Tanaka, K.; Kaji, H.; Adachi, C. J. Phys. Chem. C 2015, 119, 26283.
[21] Zhang, Q.; Kuwabara, H.; Potscavage, W. J., Jr.; Huang, S.; Hatae, Y.; Shibata, T.; Adachi, C. J. Am. Chem. Soc. 2014, 136, 18070.
[22] Shizu, K.; Tanaka, H.; Uejima, M.; Sato, T.; Tanaka, K.; Kaji, H.; Adachi, C. J. Phys. Chem. C 2015, 119, 1291.
[23] Chen, X. K.; Tsuchiya, Y.; Ishikawa, Y.; Zhong, C.; Adachi, C.; Bredas, J. L. Adv. Mater. 2017, 29, 1702767.
[24] Samanta, P. K.; Kim, D.; Coropceanu, V.; Bredas, J. L. J. Am. Chem. Soc. 2017, 139, 4042.
[25] Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234.
[26] Wang, Y.-K.; Wu, S.-F.; Yuan, Y.; Li, S.-H.; Fung, M.-K.; Liao, L.-S.; Jiang, Z.-Q. Org. Lett. 2017, 19, 3155.
[27] Kawasumi, K.; Wu, T.; Zhu, T.; Chae, H. S.; Van Voorhis, T.; Baldo, M. A.; Swager, T. M. J. Am. Chem. Soc. 2015, 137, 11908.
[28] Lee, S. Y.; Yasuda, T.; Park, I. S.; Adachi, C. Dalton. Trans. 2015, 44, 8356.
[29] Guo, J.; Li, X. L.; Nie, H.; Luo, W.; Gan, S.; Hu, S.; Hu, R.; Qin, A.; Zhao, Z.; Su, S. J.; Tang, B. Z. Adv. Funct. Mater. 2017, 27, 1606458.
[30] Shizu, K.; Noda, H.; Tanaka, H.; Taneda, M.; Uejima, M.; Sato, T.; Tanaka, K.; Kaji, H.; Adachi, C. J. Phys. Chem. C 2015, 119, 26283.
[31] Li, Y.; Liang, J.-J.; Li, H.-C.; Cui, L.-S.; Fung, M.-K.; Barlow, S.; Marder, S. R.; Adachi, C.; Jiang, Z.-Q.; Liao, L.-S. J. Mater. Chem. C 2018, 6, 5536.
[32] Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Adv. Mater. 2014, 26, 7931.
/
| 〈 |
|
〉 |