

内生真菌叶下珠生拟茎点霉的次级代谢产物研究
收稿日期: 2018-12-06
修回日期: 2019-01-09
网络出版日期: 2019-01-31
基金资助
国家自然科学基金(No.31600271)、广东省科技计划(No.2107A020211023)和广州市珠江科技新星(No.201806010080)资助项目.
Cytotoxic Secondary Metabolites from an Endophytic Fungal Strain of Phomopsis phyllanthicola
Received date: 2018-12-06
Revised date: 2019-01-09
Online published: 2019-01-31
Supported by
Project supported by the National Natural Science Foundation of China (No. 31600271), the Guangdong Provincial Project for Science and Technology (No. 2107A020211023), and the Pearl River Science and Technology New Star Fund of Guangzhou City (No. 201806010080).
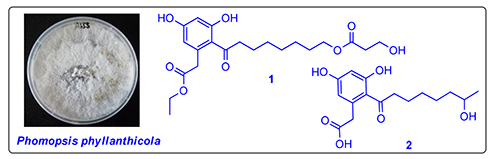
从广藿香内生真菌叶下珠生拟茎点霉(Phomopsis phyllanthicola A658)中分离纯化得到6个聚酮类化合物,分别鉴定为dothiorelone O(1),2-[3,5-二羟基-2-(7-羟基辛酰基)苯基]乙酸(2),dothiorelone M(3),dothiorelone K(4),cytosporone B(5),和cytosporone C(6).其中化合物1为新化合物,化合物2为新天然产物,新化合物的结构进一步通过HR-ESIMS,1D NMR和2D NMR等光谱技术确定.此外,对分离所得的化合物进行了体外细胞毒测试,结果表明化合物3~5显示出不同程度的细胞毒活性.
刘洪新 , 李浩华 , 陈玉蝉 , 谭海波 , 章卫民 . 内生真菌叶下珠生拟茎点霉的次级代谢产物研究[J]. 有机化学, 2019 , 39(5) : 1475 -1478 . DOI: 10.6023/cjoc201812009
The chemical investigation of the broth extract of the endophytic fungus Phomopsis phyllanthicola A658, which was isolated from the medicinal plant Pogostemon cablin, resulted in the isolation of one new compound, named dothiorelone O (1) and one new natural product 2-(3,5-dihydroxy-2-(7-hydroxyoctanoyl)phenyl)acetic acid (2), along with four known derivatives. Their structures were fully characterized by means of detailed spectroscopic analysis for new structures, and in comparison with published data for known compounds. Moreover, all of the compounds were evaluated for in vitro cytotoxic activities against SF-268, MCF-7, HepG-2, and A549 tumor cell lines.

[1] Udayanga, D.; Liu, X.; McKenzie, E. H. C.; Chukeatirote, E.; Bahkali, A. H. A.; Hyde, K. D. Fungal Divers. 2011, 50, 189.
[2] Lin, T.; Lin, X.; Lu, C. H.; Hu, Z. Y.; Huang, W. Y.; Huang, Y. J.; Shen, Y. M. Eur. J. Org. Chem. 2009, 2009, 2975.
[3] Isaka, M.; Jaturapat, A.; Rukseree, K.; Danwisetkanjana, K.; Tanticharoen, M.; Thebtaranonth, Y. J. Nat. Prod. 2001, 64, 1015.
[4] Yang, H. Y.; Gao, H. Y.; Niu, D. Y.; Yang, L. Y.; Gao, X. M.; Du, G.; Hu, Q. F. Fitoterapia 2013, 91, 189.
[5] Dai, J. Q.; Krohn, K.; Gehle, D.; Kock, I.; Florke, U.; Aust, H. J.; Draeger, S.; Schulz, B. J.; Rheinnheimer, J. Eur. J. Org. Chem. 2005, 2005, 4009.
[6] Bunyapaiboonsri, T.; Yoiprommarat, S.; Srikitikulchai, P.; Sricho- mthong, K.; Lumyong, S. J. Nat. Prod. 2010, 73, 55.
[7] Talontsi, F. M.; Islam, M. T.; Facey, P.; Douanla-Meli, C.; von Tiedemann, A.; Laatsh, H. Phytochemistry Lett. 2012, 5, 657.
[8] Yang, Z.; Ding, J.; Ding, K.; Chen, D. J.; Cen, S.; Ge, M. Phytochem. Lett. 2013, 6, 257.
[9] Adelin, E.; Servy, C.; Cortial, S.; Levaique, H.; Martin, M. T.; Retaileau, P.; Goff, G. L.; Bussaban, B.; Lumyong, S.; Ouazzani, J. Phytochemistry 2011, 72, 2406.
[10] Li, Y. Y.; Wang, M. Z.; Huang, Y. J.; Shen, Y. M. Mycology 2010, 1, 254.
[11] Wu, S. H.; Chen, Y. W.; Shao, S. C.; Wang, L. D.; Li, Z. Y.; Yang, L. Y.; Li, S. L.; Huang, R. J. Nat. Prod. 2008, 71, 731.
[12] Tan, Q. F.; Yan, X. F.; Lin, X.; Huang, Y. J.; Zheng, Z. H.; Song, S. Y.; Lu, C. H.; Shen, Y. M. Helv. Chim. Acta 2007, 90, 1811.
[13] Liu, H. X.; Liu, W. Z.; Chen, Y. C.; Sun, Z. H.; Tan, Y. Z.; Li, H. H.; Zhang, W. M. J. Asian Nat. Prod. Res. 2016, 18, 684.
[14] Wang, M.; Sun, Z. H.; Chen, Y. C.; Liu, H. X.; Li, H. H.; Tan, G. H.; Li, S. N.; Guo, X. L.; Zhang, W. M. Fitoterapia 2016, 110, 77.
[15] Liu, H. X.; Tan, H. B.; Liu, Y.; Chen, Y. C.; Li, S. N.; Sun, Z. H.; Li, H. H.; Qiu, S. X.; Zhang, W. M. Fitoterapia 2017, 117, 1.
[16] Wang, Y.; Liu, H. X.; Chen, Y. C.; Sun, Z. H.; Li, H. H.; Li, S. N.; Yan, M. L.; Zhang, W. M. Molecules 2017, 22, 765.
[17] Liu, Z.; Zhao, J. Y.; Liang, X.; Lv, X. X.; Li, Y.; Qu, J.; Liu, Y. B. Fitoterapia 2018, 127, 7.
[18] Brady, S. F.; Wagenaar, M. M.; Singh, M. P.; Janso, J. E.; Clardy, J. Org. Lett. 2000, 2, 4043.
[19] Kabayashi, A.; Hino, T.; Uneyama, K.; Kawazu, K. Symp. Chem. Nat. Prod. 1985, 27, 343.
[20] Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J. T.; Bokesch, H.; Kenney, S.; Boyd, M. R. J. Natl. Cancer Inst. 1990, 82, 1107.
/
| 〈 |
|
〉 |