

钯催化下稳定磷叶立德与烯丙基醇的脱水偶联反应
收稿日期: 2018-12-31
修回日期: 2019-01-27
网络出版日期: 2019-02-22
基金资助
河南省高等学校重点科研项目(No.19B150018)、信阳师范学院“南湖学者奖励计划”青年项目和信阳师范学院青年骨干教师资助计划(No.2018GGJS-05)资助项目.
Palladium-Catalyzed Dehydrative Cross Couplings of Stabilized Phosphorus Ylides with Allylic Alcohols
Received date: 2018-12-31
Revised date: 2019-01-27
Online published: 2019-02-22
Supported by
Project supported by the Scientific Research Project of Henan Province (No. 19B150018), the Nanhu Scholars Program for Young Scholars of Xinyang Normal University and the Young Core Instructor Program of Xinyang Normal University (No. 2018GGJS-05).
马献涛 , 于静 , 马瑞甜 , 燕然 , 张振雷 . 钯催化下稳定磷叶立德与烯丙基醇的脱水偶联反应[J]. 有机化学, 2019 , 39(3) : 830 -835 . DOI: 10.6023/cjoc201812051
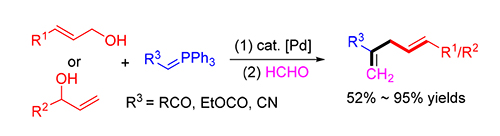
A dehydrative cross coupling of ketone-stabilized phosphorus ylides with the readily available allylic alcohols followed by an one-pot Wittig reaction is developed. A range of functional 1,4-dienes could be obtained in 52%~95% isolated yields in the presence of 5 mol% Pd(PPh3) 4 and 20 mol% B(OH) 3. The same method can be extended to ester or nitrile-stabi-lized phosphorus ylides, affording the corresponding 1,4-dienes in moderate yields.
[1] (a) Jie, M. S. F. L. K.; Pasha, M. K.; Syed-Rahmatulla, M. S. K. Nat. Prod. Rep. 1997, 14, 163.
(b) Fürstner, A.; Nevado, C.; Waser, M.; Tremblay, M.; Chevrier, C.; Teplý, F.; Aïssa, C.; Moulin, E.; Müller, O. J. Am. Chem. Soc. 2007, 129, 9150.
(c) Wilson, M. C.; Nam, S.-J.; Gulder, T. A. M.; Kauffman, C. A.; Jensen, P. R.; Fenical, W.; Moore, B. S. J. Am. Chem. Soc. 2011, 133, 1971.
[2] (a) Macklin, T. K.; Micalizio, G. C. Nat. Chem. 2010, 2, 638.
(b) Sharma, R. K.; RajanBabu, T. V. J. Am. Chem. Soc. 2010, 132, 3295.
(c) Trost, B. M.; Luan, X. J. Am. Chem. Soc. 2011, 133, 1706.
(d) McCammant, M. S.; Liao, L.; Sigman, M. S. J. Am. Chem. Soc. 2013, 135, 4167.
(e) Jin, W.; Yang, Q.; Wu, P.; Chen, J.; Yu, Z. Adv. Synth. Catal. 2014, 360, 2097.
[3] (a) Miyaura, N.; Yano, T.; Suzuki, A. Tetrahedron Lett. 1980, 21, 2865.
(b) Kabalka, G. W.; Al-Masum, M. Org. Lett. 2006, 8, 11.
(c) Lee, Y.; Akiyama, K.; Gillingham, D. G.; Brown, M. K.; Hoveyda, A. H. J. Am. Chem. Soc. 2008, 130, 446.
(d) Akiyama, K.; Gao, F.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2010, 49, 419.
(e) Gao, F.; Lee, K. P.; McGrath, Y.; Hoveyda, A. H. J. Am. Chem. Soc. 2010, 132, 14315.
(f) Gao, F.; Carr, J. L.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2012, 51, 6613.
(g) Huang, Y.; Fañanás-Mastral, M.; Minnaard, A. J.; Feringa, B. L. Chem. Commun. 2013, 49, 3309.
(h) Hamilton, J. Y.; Sarlah, D.; Carreira, E. M. J. Am. Chem. Soc. 2013, 135, 994.
(i) Gao, F.; Carr, J. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2014, 136, 2149.
(j) Sidera, M.; Fletcher, S. P. Chem. Commun. 2015, 51, 5044.
(k) Zhurkin, F. E.; Hu, X. J. Org. Chem. 2016, 81, 5795.
(l) Yang, B.; Wang, Z.-X. J. Org. Chem. 2017, 82, 4542.
[4] (a) Cornella, J.; Zarate, C.; Martin, R. Chem. Soc. Rev. 2014, 43, 8081.
(b) Matsubara, R.; Jamison, T. F. J. Am. Chem. Soc. 2010, 132, 6880.
(c) Matsubara, R.; Jamison, T. F. Chem. Asian J. 2011, 6, 1860.
(d) Ye, K.-Y.; He, H.; Liu, W.-B.; Dai, L.-X.; Helmchen, G.; You, S.-L. J. Am. Chem. Soc. 2011, 133, 19006.
(e) Hamilton, J. Y.; Sarlah, D.; Carreira, E. M. J. Am. Chem. Soc. 2014, 13 6, 2006.
(f) Gumrukcu, Y.; de Bruin, B.; Reek, J. N. H. Chem.-Eur. J. 2014, 20, 10905.
[5] (a) Thadani, A. N.; Rawal, V. H. Org. Lett. 2002, 4, 4317.
(b) Chen, X.; Chen, D.; Lu, Z.; Kong, L.; Zhu, G.-G. J. Org. Chem. 2011, 76, 6338.
(c) Wen, Y.; Jiang, H.-F. Tetrahedron Lett. 2013, 54, 4034.
(d) Todd, D. P.; Thompson, B. B.; Nett, A. J.; Montgomery, J. J. Am. Chem. Soc. 2015, 137, 12788
(e) Mateos, J.; Rivera-Chao, E.; Fañanás-Mastral, M. ACS Catal. 2017, 7, 5340.
[6] For rare examples for terminal skipped dienes synthesis, see:(a) Basavaiah, D.; Kumaragurubaran, N.; Sharada, D. S. Tetrahedron Lett. 2001, 42, 85.
(b) Basavaiah, D.; Sharada, D. S.; Kumaragurubaran, N.; Reddy, R. M. J. Org. Chem. 2002, 67, 7135.
(c) Li, Y.-Q.; Wang, H.-J.; Huang, Z.-Z. J. Org. Chem. 2016, 81, 4429.
[7] For reviews, see:(a) Edmonds, M.; Abell, A. In Modern Carbonyl Olefination, Ed.:Takeda, T., Wiley-VCH, Weinheim, Germany, 2004, pp. 1~17.
(b) Ju, Y. In Modern Organic Reactions, Vol. 3, Eds.:Hu, Y.-F.; Lin, G.-Q., Chemical Industry Press, Beijing, 2008, pp. 413~460(in Chinese). (巨勇, 现代有机反应, 主编:胡跃飞, 林国强, 第3卷, 化学工业出版社, 北京, 2008, pp. 413~460.)
(c) Gu, Y.; Tian, S.-K. Top. Curr. Chem. 2012, 327, 197.
[8] (a) Liu, W.-B.; He, H.; Dai, L.-X.; You, S.-L. Chem.-Eur. J. 2010, 16, 7376.
(b) Ma, X.-T.; Wang, Y.; Dai, R.-H.; Liu, C.-R.; Tian, S.-K. J. Org. Chem. 2013, 78, 11071.
[9] For reviews, see:(a) Bandini, M. Angew. Chem., Int. Ed. 2011, 50, 994.
(b) Sundararaju, B.; Achard, M.; Bruneau, C. Chem. Soc. Rev. 2012, 41, 4467.
(c) Butta, N. A.; Zhang, W. Chem. Soc. Rev. 2015, 44, 7929.
(d) Ferraccioli, R.; Pignataro, L. Curr. Org. Chem. 2015, 19, 106. For selected recent examples:
(e) Shen, D.; Chen, Q.; Yan, P.; Zeng, X.; Zhong, G. Angew. Chem., Int. Ed. 2017, 129, 3290.
(f) Wu, F.-P.; Peng, J.-B.; Fu, L.-Y.; Qi, X.; Wu, X.-F. Org. Lett 2017, 19, 5474.
(g) Su, Y.-L.; Han, Z.-Y.; Li, Y.-H.; Gong, L.-Z. ACS Catal. 2017, 7, 7917.
(h) Jia, X.-G.; Guo, P.; Duan, J.; Shu, X.-Z. Chem. Sci. 2018, 9, 640.
[10] Ma, X.; Yu, J.; Han, C.; Zhou, Q.; Ren, M.; Li, L.; Tang, L. Adv. Synth. Catal. 2019, https://doi.org/10.1002/adsc.201801266.
[11] (a) Ma, X.-T.; Dai, R.-H.; Zhang, J.; Gu, Y.; Tian, S.-K. Adv. Synth. Catal. 2014, 356, 2984.
(b) Ma, X.; Yu, L.; Su, C.; Yang, Y.; Li, H.; Xu, Q. Adv. Synth. Catal. 2017, 359, 1649.
(c) Ma, X.; Xu, Q.; Li, H.; Su, C.; Yu, L.; Zhang, X.; Cao, H.; Han, L.-B. Green Chem. 2018, 20, 3408.
(d) Ma, X.; Su, C.; Xu, Q. Top. Curr. Chem. 2016, 374, 27.
[12] Kaszynski, P.; Friedli, A. C.; Michl, J. J. Am. Chem. Soc. 1992,114, 601.
[13] Liu, H.-J.; Wynn, H. Tetrahedron Lett. 1982, 23, 3151.
/
| 〈 |
|
〉 |