

无溶剂的β-卟啉甲醛与酮的Aldol反应
收稿日期: 2018-10-05
修回日期: 2019-01-18
网络出版日期: 2019-03-08
基金资助
河南省科技攻关(No.192102310141)资助项目.
Solvent-Free Aldol Reaction of β-Porphyrin Formaldehyde with Ketone
Received date: 2018-10-05
Revised date: 2019-01-18
Online published: 2019-03-08
Supported by
Project supported by the Science and Technology Planning Project of Henan Province of China (No. 192102310141).
周详 , 周菲 , 贾晓良 , 于佳伟 , 申琦 , 张运晓 , 石伟民 . 无溶剂的β-卟啉甲醛与酮的Aldol反应[J]. 有机化学, 2019 , 39(7) : 2084 -2088 . DOI: 10.6023/cjoc201810004
A convenient approach for the synthesis of β-substituted α,β-unsaturated carbonyl porphyrin compounds via base-catalyzed aldol reaction was developed. By this method, a series of β-substituted α,β-unsaturated carbonyl porphyrin compounds were constructed using β-porphyrin formaldehyde and ketones with moderate to excellent yields under mild reaction conditions, especially solvent-free, and good functional group tolerance. Furthermore, this process was successfully applied to the reactions of different metal porphyrin which have been reported to have poor reaction effects with good yields.
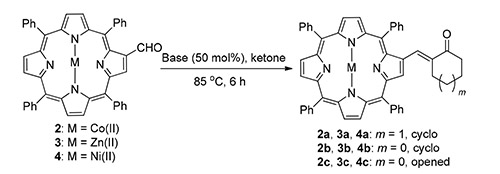
Key words: metal porphyrin; α,β-unsaturated carbonyl; aldol reaction; solvent-free
[1] Hammerer, F.; Garcia, G.; Chen, S.; Poyer, F.; Achelle, S.; Fiorini-Debuisschert, C.; Teulade-Fichou, M. P.; Maillard, P. J. Org. Chem. 2014, 79, 1406.
[2] Hammerer, F.; Poyer, F.; Fourmois, L.; Chen, S.; Garcia, G.; Teulade-Fichou, M. P.; Maillard, P.; Mahuteau-Betzer, F. Bioorg. Med. Chem. 2018, 26, 107.
[3] Wen, D. D.; Yue, F. Y.; Liu, W. W.; Chen, S. Q.; Chen, X. Z. J. Alloys Compd. 2018, 3, 217.
[4] Temelli, B.; Ozasik, O.; Yüksel, D. Eur. J. Org. Chem. 2017, 2017, 4905.
[5] Sean, A. V.; David, I. S.; Dirk, M. G.; Marja, I.; Nikolai, T.; Lemmetyinen, H.; Palkar, A.; Echegoyen, L.; Chen, X. H.; John, Z.; Zhang, H. J. Phys. Chem. B 2006, 110. 14155.
[6] Yedukondalu, M.; Maity, D. K.; Ravikanth, M. Eur. J. Org. Chem. 2010, 1544.
[7] Zeng, J. F.; Yang, W. D.; Shi, D. J.; Li, X. J.; Zhang, H. J.; Chen, M. Q. ACS Biomater. Sci. Eng. 2018, 4, 963.
[8] Zhao, R. R.; Liang, X. L.; Zhao, B.; Chen, M.; Liu, R. F.; Sun, S. J.; Yue, X. L.; Wang, S. M. Biomaterials 2018, 173, 58.
[9] Kadish, K. M.; Shao, J.; Ou, Z.; Comte, C.; Gros, C. P.; Guilard, R. J. Porphyrins Phthalocyanines 2000, 4, 639.
[10] Sutton, J. M.; Fernandez, N.; Boyle, R. W. J. Porphyrins Phthalocyanines 2000, 4, 655.
[11] Jiang, X. L.; Li, P. L.; Wang, Y. C.; Shen, Q.; Tao, J. C.; Shi, W. M. Chin. J. Chem. 2012, 30, 405.
[12] Borodin, A. J. Prakt. Chem. 1864, 93, 413.
[13] Wurtz, A. J. Prakt. Chem. 1872, 5, 457.
[14] Johnson, J. S.; Evans, D. A. Acc. Chem. Res. 2000, 33, 325.
[15] (a) Moura, N. M. M.; Mariz, I. F. A.; Cavaleiro, J. A. S.; Silva, A. M. S.; Lodeiro, C.; Martinho, J. M. G.; Macoas, E. M. S.; Neves, M. G. P. M. S. J. Org. Chem. 2018, 83, 5282.
(b) Zhang, Q.; Jia, X. S.; Yin, L. Tetrahedron 2018, 11, 041.
(c) Kan, S. B. J.; Ng, K. K. H.; Paterson, I. Angew. Chem., Int. Ed. 2013, 52, 9097.
[16] (a) Ishkov, Y. V.; Zhilina, I. Z.; Barday, L. P. J. Porphyrins Phthalocyanines 2003, 7, 761.
(b) Ishkov, Y. V.; Zhilina I. Z.; Bardai, L. P.; Vodzinskii, S. V. Russ. J. Org. Chem. 2004, 40, 434.
[17] Yu, J. W. M.S. Thesis, Zhengzhou University, Zhengzhou, 2014 (in Chinese). (于佳伟, 硕士论文, 郑州大学, 郑州, 2014.)
/
| 〈 |
|
〉 |