

通过[2,3]-Wittig重排合成烯丙基-甲基-N-泛酸酰胺
收稿日期: 2018-11-05
修回日期: 2019-01-17
网络出版日期: 2019-03-08
基金资助
国家自然科学基金(Nos.21372205,21302175)和河南省科技厅基础研究(No.132300410028)资助项目.
A Synthetic Route to Access Allyl-methyl-N-pantothenamide via [2,3]-Wittig Rearrangement
Received date: 2018-11-05
Revised date: 2019-01-17
Online published: 2019-03-08
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21372205, 21302175) and the Basic Research Project of Science and Technology Department of Henan Province (No. 132300410028).
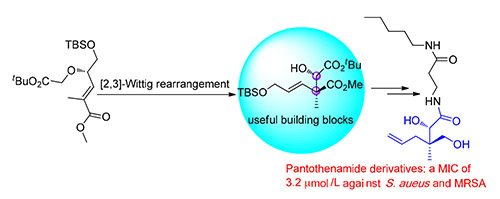
甲基-烯丙基-N-泛酸酰胺(1)具有手性季碳和邻位仲醇结构片段的抗菌剂,可以巧妙通过[2,3]-Wittig重排和钯催化的甲酸还原构建了其分子骨架,以10步反应制备了1.
关键词: [2,3]-Wittig重排; N-Pantothenamide; 手性季碳; 抗菌剂
夏礼文 , 赵青 , 巴梦雨 , 胡超平 , 孙默然 , 杨华 . 通过[2,3]-Wittig重排合成烯丙基-甲基-N-泛酸酰胺[J]. 有机化学, 2019 , 39(7) : 2035 -2041 . DOI: 10.6023/cjoc201811009
The synthetic route of allyl-methyl-N-pantothenamide (1) featuring[2,3]-Wittig rearrangement and palladium catalyzed formate reduction to assemble the requisite quaternary carbon with adjacent secondary alcohol has been reported. Our strategy presents a facile synthetic route to access 1 in 10 steps, which also provide a novel inspiration to construct chiral quaternary carbon via asymmetrical[2,3]-Wittig rearrangement.

[1] Clifton, G.; Bryant, S. R.; Skinner, C. G. Arch. Biochem. Bioph. 1970, 137, 523.
[2] (a) Zhang, Y.-M.; Frank, M. W.; Virga, K. G.; Lee, R. E.; Rock, C. O.; Jackowski, S. J. Biolog. Chem. 2004, 279, 50969.
(b) Maršavelski, A. RSC Adv. 2016, 6, 44888.
[3] Hoegl, A.; Darabi, H.; Tran, E.; Awuah, E.; Kerdo, E. S. C.; Habib, E. Biorg. Med. Chem. Lett. 2014, 24, 3274.
[4] Akinnusi, T. O.; Vong, K.; Auclair, K. Bioorg. Med. Chem. 2011, 19, 2696.
[5] Fráter, G. Tetrahedron Lett. 1981, 22, 425
[6] Tsao, K.-W.; Cheng, C.-Y.; Isobe, M. Org. Lett. 2012, 14, 5274
[7] Hiersemann, M.; Lauterbach, C.; Pollex, A. Eur. J. Org. Chem. 1999, 2713.
[8] Hiersemann, M.; Abraham, L.; Pollex, A. Synlett 2003, 1088.
[9] (a) Yang, H.; Sun, M.; Zhao, S.; Zhu, M.; Xie, Y.; Niu, C. J. Org. Chem. 2013, 78, 339.
(b) Sun, M.; Dai, L; Yang, H.; Liu, H.; Yu, D. Chin. J. Org. Chem. 2018, 38, 2443(in Chinese). (孙默然, 代磊, 杨华, 刘宏民, 于德泉, 有机化学, 2018, 38, 2443.)
(c) Zhou, H.; Sun, M.; Cao, Q.; Bai, L.; Xie, Y.; Yang, H. Chin. J. Org. Chem. 2013, 33, 2515(in Chinese). (周航, 孙默然, 曹其伟, 朱明, 白磊阳, 谢阳娜, 杨华, 有机化学, 2013, 33, 2515.)
[10] Marshall, J. A.; Lebreton, J. J. Am. Chem. Soc. 1988, 110, 2925.
[11] (a) Hirokawa, Y.; Kitamura, M.; Maezaki, N. Tetrahedron:Asymmetry 2008, 19, 1167.
(b) Barrett, I. M.; Breeden, S. W. Tetrahedron:Asymmetry 2004, 15, 3015.
[12] Seco, J. M.; Quiñoá, E.; Riguera, R. Tetrahedron:Asymmetry 2001, 12, 2915.
[13] (a) Osamu, T.; Koichi, M.; Takeshi, N. Chem. Lett. 1987, 16, 69.
(b) Fujimoto, K, Nakai, T. Tetrahedron Lett. 1994, 35, 5019.
[14] Diéguez, H. R.; López, A.; Domingo, V.; Arteaga, J. F.; Dobado, J. A.; Herrador, M. M. J. Am. Chem. Soc. 2010, 132, 254.
[15] Hughes, G.; Lautens, M.; Wen, C. Org. Lett. 2000, 2, 107.
[16] Vong, K. K. H.; Maeda, S.; Tanaka, K. Chem.-Eur. J. 2016, 22, 18865.
/
| 〈 |
|
〉 |