

N,O-双齿螯合作用下铜促进的C-H键直接硝基化反应
收稿日期: 2019-01-11
修回日期: 2019-02-25
网络出版日期: 2019-03-08
基金资助
国家自然科学基金(Nos.21772179,21672192)和河南省高校科技创新人才计划(No.19HASTIT038)资助项目.
Copper-Promoted Direct Nitration of Arenes Assisted by an N,O-Bidentate Directing System
Received date: 2019-01-11
Revised date: 2019-02-25
Online published: 2019-03-08
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21772179, 21672192), the Program for Science & Technology Innovation Talents in Universities of Henan Province (No. 19HASTIT038).
王云龙 , 张林宝 , 牛俊龙 , 宋毛平 . N,O-双齿螯合作用下铜促进的C-H键直接硝基化反应[J]. 有机化学, 2019 , 39(6) : 1761 -1766 . DOI: 10.6023/cjoc201901015
Cu(Ⅱ)-promoted C-H nitration of arenes has been disclosed with the aid of N,O-bidentate directing group. The protocol was operationally simple by using NaNO2as the nitration source. Various amide substrates were tolerated in the reaction system, which establishes opportunities for developing simple and facile methods, and enriches the strategies to access aromatic nitro derivatives.
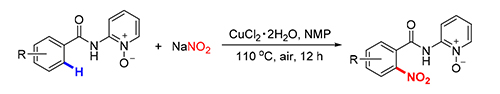
Key words: C-H activation; bidentate direction; Cu-catalyzed; nitration
[1] (a) Olah, G. A.; Malhotra, R.; Narang, S. C. Nitration:Methods and Mechanisms, Wiley-VCH, Weinheim, 1989.
(b) Feuer, H.; Nielsen, A. T. Nitro Compounds:Recent Advances in Synthesis and Chemistry, Wiley-VCH, Weinheim, 1990.
[2] (a) Schofield, K. Aromatic Nitrations, Cambridge University Press, Cambridge, 1980.
(b) Ono, N. The Nitro Group in Organic Synthesis, Wiley-VCH, Weinheim, 2001.
[3] Hearn, R.; Russell, M. K. R. G. Ann. Rheum. Dis. 1983, 42(Suppl.), 39.
[4] (a) Tani, K.; Lukin, K.; Eaton, P. E. J. Am. Chem. Soc. 1997, 119, 6.
(b) Salzbrunn, S.; Simon, J.; Prakash, G. K. S.; Petasis, N. A.; Olah, G. A. Synlett 2000, 1485.
(c) Prakash, G. K. S.; Panja, C.; Mathew, T.; Surampudi, V.; Petasis, N. A.; Olah, G. A. Org. Lett. 2004, 6, 2205.
(d) Yan, G.; Yang, M. Org. Biomol. Chem. 2013, 11, 2554.
(e) Das, J. P.; Sinha, P.; Roy, S. Org. Lett. 2002, 4, 3055.
[5] (a) Kakiuchi, F.; Chatani, N. Adv. Synth. Catal. 2003, 345, 1077.
(b) Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792.
(c) Satoh, T.; Miura, M. Chem.-Eur. J. 2010, 16, 11212.
(d) Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. Chem. Soc. Rev. 2011, 40, 4740.
(e) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem. Int. Ed. 2009, 48, 5094.
(f) Ackermann, L. Acc. Chem. Res. 2014, 47, 281.
(g) Misal Castro, L. C.; Chatani, N. Chem. Lett. 2015, 44, 410.
(h) Satoh, T.; Miura, M. Chem. Lett. 2006, 36, 200.
[6] (a) Liu, Y.-K.; Lou, S.-J.; Xu, D.-Q.; Xu, Z.-Y. Chem.-Eur. J. 2010, 13590.
(b) Zhang, W.; Lou, S.; Liu, Y.; Xu, Z. J. Org. Chem. 2013, 78, 5932.
(c) Zhang, L.; Liu, Z.; Li, H.; Fang, G.; Barry, B. D.; Belay, T. A.; Bi, X.; Liu, Q. Org. Lett. 2011, 13, 6536.
(d) Zhang, H.; Zhao, L.; Wang, D.-X.; Wang, M.-X. Org. Lett. 2013, 15, 3836.
(e) Xie, F.; Qi, Z.; Li, X. Angew. Chem., Int. Ed. 2013, 52, 11862.
(f) Hernando, E.; Castillo, R. R.; Rodriguez, N.; Arrayás, R. G.; Carretero, J. C. Chem. Eur. J. 2014, 20, 13854.
(g) Kianmehr, E.; Nasab, S. B. Eur. J. Org. Chem. 2018, 6447.
(h) Katayev, D.; Pfister, K. F.; Wendling, T.; Gooβen, L. J. Chem.-Eur. J. 2014, 20, 9902.
(i) Liu, J.; Zhuang, S.; Gui, Q.; Chen, X.; Yang, Z.; Tan, Z. Adv. Synth. Catal. 2015, 357, 732.
[7] Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 13154.
[8] Selected examples.
(a) Asako, S.; Ilies, L.; Nakamura, E. J. Am. Chem. Soc. 2013, 135, 17755.
(b) Shang, R.; Ilies, L.; Matsumoto, A.; Nakamura, E. J. Am. Chem. Soc. 2013, 135, 6030.
(c) Fruchey, E. R.; Monks, B. M.; Cook, S. P. J. Am. Chem. Soc. 2014, 136, 13130.
[9] Selected examples.
(a) Grigorjeva, L.; Daugulis, O. Angew. Chem., Int. Ed. 2014, 53, 10209.
(b) Grigorjeva, L.; Daugulis, O. Org. Lett. 2014, 16, 4684.
(c) Grigorjeva, L.; Daugulis, O. Org. Lett. 2014, 16, 4688.
(d) Ma, W.; Ackermann, L. ACS Catal. 2015, 5, 2822.
(e) Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, Z.-J.; Zheng, X.-X.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Angew. Chem., Int. Ed. 2015, 54, 272.
(f) Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Zheng, X.-X.; Liu, Z.-J.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Angew. Chem., Int. Ed. 2015, 54, 10012.
(g) Saxena, P.; Kapur, M. Chem. Asian J. 2018, 13. 861.
[10] Selected examples:
(a) Aihara, Y.; Chatani, N. J. Am. Chem. Soc. 2014, 136, 898.
(b) Song, W.; Lackner, S.; Ackermann, L. Angew. Chem., Int. Ed. 2014, 53, 2477.
(c) Shiota, H.; Ano, Y.; Aihara, Y.; Fukumoto, Y.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 14952.
[11] Selected examples.
(a) Tran, L. D.; Popov, I.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 18237.
(b) Truong, T.; Klimovica, K.; Daugulis, O. J. Am. Chem. Soc. 2013, 135, 9342.
(c) Tran, L. D.; Roane, J.; Daugulis, O. Angew. Chem., Int. Ed. 2013, 52, 6043.
(d) Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842.
(e) Shang, M.; Sun, S.-Z.; Wang, H.-L.; Laforteza, B. N.; Dai, H.-X.; Yu, J.-Q. Angew. Chem., Int. Ed. 2014, 53, 10439.
(f) Shang, M.; Wang, H.-L.; Sun, S.-Z.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2014, 136, 11590.
(g) Li, X.; Liu, Y.-H.; Gu, W.-J.; Li, B.; Chen, F.-J.; Shi, B.-F. Org. Lett. 2014, 16, 3904.
(h) Dong, J.; Wang, F.; You, J. Org. Lett. 2014, 16, 2884.
(i) Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Angew. Chem., Int. Ed. 2013, 52, 4457.
(j) Chen, F.-J.; Liao, G.; Li, X.; Wu, J.; Shi, B.-F. Org. Lett. 2014, 16, 5644.
(k) Rouquet, G.; Chatani, N. Angew. Chem., Int. Ed. 2013, 52, 11726.
(l) Zhang, Q.; Chen, K.; Shi, B.-F. Synlett 2014, 25, 1941.
(m) Daugulis, O.; Roane, J.; Tran, L. D. Acc. Chem. Res. 2015, 48, 1053.
(n) Dou, Y.-D.; Yin, B.; Zhang, P.-F.; Zhu, Q. Eur. J. Org. Chem. 2018, 4571.
(o) Wang, C.-M.; Tang, K.-X.; Gao, T.-H.; Chen, L.; Sun, L.-P. J. Org. Chem. 2018, 83, 8315.
(p) Tu, D.-Q.; Luo, J.; Jiang, C. Chem. Commun. 2018, 54, 2514.
(q) Vinayak, B.; Chandrasekharam, M. Org. Lett. 2017, 19, 3528.
[12] Selected our recent reports:(a) Hao, X.-Q.; Chen, L.-J.; Ren, B.; Li, L.-Y.; Yang, X.-Y.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Org. Lett. 2014, 16, 1104.
(b) Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, K.; Ren, B.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. J. Org. Chem. 2014, 79, 10399.
[13] Selected examples.
(a) Wang, D.-W.; Zhao, K.-Y.; Xu, C.-Y.; Miao, H.-Y.; Ding, Y.-Q. ACS Catal. 2014, 4, 3910.
(b) Wang, D.-W.; Ge, B.-Y.; Li, L.; Shan, J.; Ding, Y.-Q. J. Org. Chem. 2014, 79, 8607.
(c) Guo, X-K.; Zhang, L.-B.; Wei, D.-H.; Niu, J.-L. Chem. Sci. 2015, 6, 7059.
(d) Wang, D.-W.; Yu, X.; Yao, W.; Hu, W.-K.; Ge, C.-Y.; Shi, X.-D. Chem.-Eur. J. 2016, 22, 55433.
(e) Yu, X.-L.; Wang, D.-S.; Xu, Z.-J.; Yang, B.-B.; Wang, D.-W. Org. Chem. Front. 2017, 4, 1011.
(f) Wu, Q.; Pan, L.; Du, G.-M.; Zhang, C.; Wang, D.-W. Org. Chem. Front. 2018, 5, 2668.
(g) Xu, Z.-J.; Yu, X.-L.; Sang, X.-X.; Wang, D.-W. Green Chem. 2018, 20, 2571.
(h) Wang, Y.; Du, C.; Wang, Y.-Y.; Guo, X.-K.; Fang, Lei.; Song, M.-P.; Niu, J.-L.; Wei, D.-H. Adv. Synth. Catal. 2018, 360, 2668.
(i) Wang, D.-W.; Yu, X.-L.; Ge, B.-Y.; Miao, H.-Y.; Ding, Y.-Q. Chin. J. Org. Chem. 2015, 35, 676.
(j) Hu, X.-Y.; Yang, B.-B.; Yao, W.; Wang, D.-W. Chin. J. Org. Chem. 2018, 38, 3296.
[14] Suess, A. M.; Ertem, M. Z.; Cramer, C. J.; Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 9797.
/
| 〈 |
|
〉 |