

基于Strecker反应的含氰基多取代吡唑的合成及生物活性研究
收稿日期: 2018-12-11
修回日期: 2019-02-28
网络出版日期: 2019-03-21
基金资助
国家自然科学基金(No.20902107)资助项目.
Synthesis and Biological Activity Study of Novel Cyano-containing Multi-substituted Pyrazoles Obtained via Strecker Reaction
Received date: 2018-12-11
Revised date: 2019-02-28
Online published: 2019-03-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 20902107).
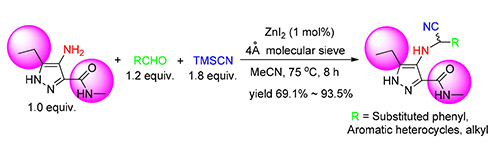
为探索多取代吡唑胺的Strecker反应及含氰基吡唑化合物的生物活性,首次实现了ZnI2催化下多取代吡唑胺、三甲基硅腈(TMSCN)和醛的Strecker反应,初步探索了反应条件及醛底物的范围.共获得20个全新含氰基多取代吡唑目标物,最高收率为93.5%,并通过核磁共振氢谱(1H NMR)、碳谱(13C NMR)和高分辨质谱(HRMS)确证了其结构.活性测试表明,10×10-3 g/L浓度下,13个化合物对蚊幼虫活性为100%,5×10-3 g/L浓度下4个化合物活性高于40%;在500×10-3 g/L浓度下10个化合物对黏虫体现一定活性.在500×10-3 g/L浓度下5个化合物对烟草花叶病毒具有一定钝化活性,最高活性为31.8%,4个化合物则具有保护活性,最高活性为28.3%.在50×10-3 mg/mL浓度下,3个化合物对瓜果腐霉菌、芦笋茎枯菌、辣椒疫霉菌、水稻纹枯菌等显示一定抑制活性,其中4-[(1-氰基十二烷基)氨基]-5-乙基-N-甲基-1H-吡唑-3-甲酰胺(3t)对辣椒疫霉活性达62.3%.这表明氨基邻位具有大位阻基团的吡唑胺底物能顺利发生Strecker反应,含氰基吡唑类目标物显示多样的生物活性,具有一定的研究价值.
苏诗淼 , 朱墨 , 张大强 , 袁德凯 . 基于Strecker反应的含氰基多取代吡唑的合成及生物活性研究[J]. 有机化学, 2019 , 39(7) : 2026 -2034 . DOI: 10.6023/cjoc201812019
In order to study the Strecker reaction of multi-substituted amino armorotic heterocycles and the biological activity of the target compounds, the reaction of 3,5-disubstituted pyrazole-4-amine, TMSCN and aldehydes was realized for the first time by the catalysis of anhydrous ZnI2 with 4Å molecular seive. The reaction condition was preliminarily optimized and the substrate scop of aldehydes was studied. 20 target compounds of cyano-containing multi-substituted pyrazoles were obtained with the highest yield of 93.5%, and the sturctures of the compounds were confirmed via 1H NMR, 13C NMR and HRMS methods. Priliminary bioassay of the target compounds showed that 13 target compounds possessed 100% larvicidal activity against mosquito at a concentration of 10×10-3 g/L, and four compounds possessed over 40% larvicidal activity at 5×10-3 g/L, and 10 compounds poccessed weak larvicidal activity against army worm at 500×10-3 g/L with the highest activity of 40%; five compounds were confirmed poccessed good inactivation activity against tobacco mosaic virus (TMV) in vivo with the highest inhibition rate of 31.8%, and four compounds possessed moderate host-protection activity against TMV in vivo with the highest rate of 28.3%; in addition, at a concentration of 50×10-3 g/L, three compounds showed moderate fungicidal activity against Pythium aphanidermatum, Phomopsis asparagi, Phytophora capsic and Rhizoctonia solani in vitro. 4-((1-cyanododecyl)amino)-5-ethyl-N-methyl-1H-pyrazole-3-carboxamide (3t) possessed the highest activity against Phytophora capsic of 62.3%. All the results showed that 3,5-disubstitued pyrazole-4-amine could undergo Strecker reaction smoothly, and this study gave a useful example to explore the Strecker reaction of other multi-substituted aromatic hetreocyclic amines. Additionally, the larvicidal and anti-TMV activity of the target compounds gave some clues in design cyano-containing biological heterocycles.

[1] (a) Strecker, D. Ann. Chem. Pharm. 1850, 75, 27.
(b) Kouznetsov, V. V.; Galvis, C. E. P. Tetrahedron 2018, 74, 773.
[2] (a) de Bruin, G.; Mock, E. D.; Hoogendoorn, S.; van den Nieuwendijk, A. M. C. H.; Mazurek, J.; van der Marel, G. A.; Florea, B. I.; Overkleeft, H. S. Chem. Commun. 2016, 52, 4064.
(b) Hirata, T.; Ueda, A.; Oba, M.; Doi, M.; Demizu, Y.; Kurihara, M.; Nagano, M.; Suemune, H.; Tanaka, M. Tetrahedron 2015, 71, 2409.
[3] (a) Song, R. Z.; Han, Z. M.; He, Q. Q.; Fan, R. H. Org. Lett. 2016, 18, 5328.
(b) Tay, G. C.; Sizemore, N.; Rychnovsky, S. D. Org. Lett. 2016, 18, 3050.
(c) Guchhait, S. K.; Priyadarshani, G.; Gulghane, N. M. RSC Adv. 2016, 6, 56056.
(d) Afraj, S. N.; Chen, C. P.; Lee, G. H. RSC Adv. 2016, 6, 29783.
[4] (a)Yashin, N. V.; Averina, E. B.; Sedenkova, K. N.; Kuznetsova, T. S.; Zefirov, N. S. Russ. Chem. Bull. 2013, 62, 928.
(b) Acena, J. L.; Sorochinsky, A. E.; Soloshonok, V. A. Synthesis 2012, 44, 1591.
[5] (a) Malins, L. R.; deGruyter, J. N.; Robbins, K. J.; Scola, P. M.; Eastgate, M. D.; Ghadiri, M. R.; Baran, P. S. J. Am. Chem. Soc. 2017, 139, 5233.
(b) Xing, J. H.; Brooks, A. F.; Fink, D.; Zhang, H. B.; Piert, M. R.; Scott, P. J. H.; Shao, X. Synlett 2017, 28, 371.
(c) Cai, H. C.; Mangner, T. J.; Muzik, O.; Wang, M. W.; Chugani, D. C.; Chugani, H. T. ACS Med. Chem. Lett. 2014, 5, 1152.
(d) Kokhan, S. O.; Tymtsunik, A. V.; Grage, S. L.; Afonin, S.; Babii, O.; Berditsch, M.; Strizhak, A. V.; Bandak, D.; Platonov, M. O.; Komarov, I. V.; Ulrich, A. S.; Mykhailiuk, P. K. Angew. Chem., Int. Ed. 2016, 55, 14788.
[6] (a) Katsuhiko, M.; Bilal, A. A.; Christopher, M. S.; Shajila, S.; Michio, K. J. Am. Chem. Soc. 2016, 138, 12975.
(b) Dhanasekaran, S.; Suneja, A.; Bisai, V.; Singh, V. K. Org. Lett. 2016, 18, 634.
(c) Chen, C. H.; Genapathy, S.; Fischer, P. M.; Chan, W. C. Org. Biomol. Chem. 2014, 12, 9764.
(d) Aleiwi, B. A.; Schneider, C. M.; Kurosu, M. J. Org. Chem. 2012, 77, 3859.
[7] (a) Brahmachari, G.; Kumar, A.; Srivastava, A. K.; Gangwar, S.; Misra, N.; Gupta, V. K.; Rajnikant, R. RSC Adv. 2015, 5, 80967.
(b) Luis, J. A. S.; De Aquino, T. M.; Lira, B.F.; Filho, P. F. A.; Scotti, M. T.; Scotti, L.; De Moura, R. O.; Mendonca, F. J. Acta Pharm. (Zagreb, Crotia) 2014, 64, 233.
[8] (a) Carreno Otero, A. L.; Vargas Mendez, L. Y.; Duque L., J. E.; Kouznetsov, V. V. Eur. J. Med. Chem. 2014, 78, 392.
(b) Yu, X. H.; Wang, G. H.; Kong, X. L.; Huang, H. Y. CN 103183707, 2013[Chem. Abstr. 2013, 159, 229714].
(c) Lamberth, C.; Dumeunier, R.; Trah, S.; Wendeborn, S.; Godwin, J.; Schneiter, P.; Corran, A. Bioorg. Med. Chem. 2013, 21, 127.
[9] (a) Parker, E. T.; Zhou, M. S.; Burton, A. S.; Glavin, D. P.; Dworkin, J. P.; Krishnamurthy, R.; Fernandez, F. M.; Bada, J. L. Angew. Chem., Int. Ed. 2014, 53, 8132.
(b) Bolm, C.; Mocci, R.; Schumacher, C.; Turberg, M.; Puccetti, F.; Hernandez, J. G. Angew. Chem., Int. Ed. 2018, 57, 2423.
(c) Riffet, V.; Frison, G.; Bouchoux, G. J. Phys. Chem. A 2018, 122, 1643.
[10] Li, P. C.; Zhang, Y. D.; Chen, Z. L.; Zhang, X. X. Tetrahedron Lett. 2017, 58, 1854.
[11] Reddy, V. V. R.; Saritha, B.; Ramu, R.; Varala, R.; Jayashree, A. Asian J. Chem. 2014, 26, 7439.
[12] Hajipour, A. R.; Dehbane, I. M. Iran. J. Catal. 2012, 2, 147.
[13] Wang, S. Y.; Xu, J. N.; Zheng, J. F.; Chen, X. D.; Shan, L.; Gao, L. J.; Wang, L.; Yu, M.; Fan, Y. Inorg. Chim. Acta 2015, 437, 81.
[14] (a) Chai, J.; Wang, P. C.; Jia, J.; Ma, B.; Sun, J.; Tao, Y. F.; Zhang, P.; Wang, L.; Fan, Y. Polyhedron 2018, 141, 369.
(b) Ibanez, S.; Poyatos, M.; Peris, E. Chem. Comm. 2017, 53, 3733.
(c) Prakash, G. K. S.; Mathew, T.; Panja, C.; Kulkarni, A.; Olah, G. A.; Harmer, M. A. Adv. Synth. Catal. 2012, 354, 2163.
[15] (a) Saravanan, S.; Khan, N. H.; Jakhar, A.; Ansari, A.; Kureshy, R. I.; Abdi, S. H. R.; Kumar, G. RSC Adv. 2015, 5, 99951.
(b) Zhu, C.; Xia, J. Bao; Chen, C. Tetrahedron Lett. 2014, 55, 232.
[16] Costantini, N. V.; Bates, A. D.; Haun, G. J.; Chang, N. M.; Moura- Letts, G. ACS Sustainable Chem. Eng. 2016, 4, 1906.
[17] Li, N. B.; Wang, J. Y.; Zhang, X. H.; Qiu, R. H.; Wang, X; Chen, J. Y.; Yin, S. F.; Xu, X. H. Dalton Trans. 2014, 43, 11696.
[18] Brahmachari, G.; Banerjee, B. Asian J. Org. Chem. 2012, 1, 251.
[19] Zhang, X. L.; Wu, Q. P.; Zhang, Q. S. J. Chem. Res. 2013, 37, 690.
[20] Ghasemnejad-Bosra, H.; Arta, R. Org. Chem.: Indian J. 2015, 11, 399.
[21] Huber, R.; Bigler, R.; Mezzetti, A. Organometallics 2015, 34, 3374.
[22] Khalafi-Nezhad, A.; Divar, M.; Panahi, F. J. Org. Chem. 2013, 78, 10902.
[23] Ghafuri, H.; Roshani, M. RSC Adv. 2014, 4, 58280.
[24] Nammalwar, B.; Fortenberry, C.; Bunce, R. A. Tetrahedron Lett. 2014, 55, 379.
[25] (a) Khazdooz, L.; Zarei, A.; Hajipour, A. R.; Sheikhan, N. Iran. J. Catal. 2012, 2, 63.
(b) Akbari, J. C. R. Chim. 2012, 15, 471.
[26] Sengupta, A.; Su, C. L.; Bao, C. L.; Nai, C. T.; Loh, K. P. ChemCatChem 2014, 6, 2507.
[27] Zhang, J.; Du, G. F.; Gu, C. Z.; Dai, B. J. Chin. Org. Chem. 2017, 37, 914(in Chinese). (张洁, 杜广芬, 顾承志, 代斌, 有机化学, 2017, 37, 914.)
[28] (a) Chen, W.; Peng, X. W.; Zhong, L. X.; Li, Y.; Sun, R. C. ACS Sustainable Chem. Eng. 2015, 3, 1366.
(b) Dabral, S.; Turberg, M.; Wanninger, A.; Bolm, C.; Hernandez, J. G. Molecules 2017, 22, 146/1.
[29] Dekamin, M. G.; Azimoshan, M.; Ramezani, L. Green Chem. 2013, 15, 811.
[30] (a) Islami, M.; Dekamin, M. G.; Motlagh, L.; Maleki, A. Green Chem. Lett. Rev. 2018, 11, 36.
(b) Reinares-Fisac, D.; Aguirre-Diaz, L. M.; Iglesias, M.; Snejko, N.; Gutierrez-Puebla, E.; Monge, M. A.; Gandara, F. J. Am. Chem. Soc. 2016, 138, 9089.
(c) Xia, J.; Xu, J. N.; Fan, Y.; Song, T. Y.; Wang, L.; Zheng, J. F. Inorg. Chem. 2014, 53, 10024.
(d) Chen, H.; Ju, J.; Meng, Q. P.; Su, J.; Lin, C.; Zhou, Z.Y.; Li, G. B.; Wang, W. L.; Gao, W. L.; Zeng, C. M.; Tang, C.; Lin, J. H.; Yang, T.; Sun, J. L. J. Am. Chem. Soc. 2015, 137, 7047.
(e) Wang, W. L.; Wang, Y.; Wu, B.; Cong, R. H.; Gao, W. L.; Qin, B.; Yang, T. Catal. Commun. 2015, 58, 174.
[31] (a) Azizi, N.; Farhadi, E. Appl. Organomet. Chem. 2018, 32, e4188.
(b) Arora, P.; Rajput, J. K.; Singh, H. RSC Adv. 2015, 5, 97212.
(c) Indalkar, K. S.; Khatri, C. K.; Chaturbhuj, G. U. Tetrahedron Lett. 2017, 58, 2144.
(d) Mobaraki, A.; Movassagh, B.; Karimi, B. ACS Comb. Sci. 2014, 16, 352.
(e) Gawande, M. B.; Rathi, A. K.; Nogueira, I. D.; Varma, R. S.; Branco, P. S. Green Chem. 2013, 15, 1895.
[32] (a) Chen, D.; Xu, M. H. J. Org. Chem. 2014, 79, 7746.
(b) Dekamin, M. G.; Mokhtari, Z. Tetrahedron 2012, 68, 922.
(c) Yang, K.; Liu, L. J.; Liu, J. T. J. Org. Chem. 2014, 79, 3215.
(d) Yuan, X. M.; Xu, J.; Liu, Z. J.; Yang, X. J.; Wang, L. M.; Zhang, Y.; Yang, X. Y.; He, X. P.; Liu, J. T. J. Fluorine Chem. 2012, 144, 102.
[33] Miao, Z. W. In Tools for Stereoselective Synthesis, Eds.:Martin, M.; Boysen, K., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013, Chapter 1, pp. 3~26.
[34] (a) Pellissier, H. Chem. Rev. 2013, 113, 442.
(b) Sashikanth, S.; Raju, V.; Somaiah, S.; Rao, P. S.; Reddy, K. V. Synthesis 2013, 45, 621.
[35] Oliveira, M. T.; Lee, J. W. ChemCatChem 2017, 9, 377.
[36] (a) Sugiishi, T.; Matsugi, M.; Hamamoto, H.; Amii, H. RSC Adv. 2015, 5, 17269.
(b) Sadhukhan, A.; Saravanan, S.; Khan, N. H.; Kureshy, R. I.; Abdi, S. H. R.; Bajaj, H. C. J. Org. Chem. 2012, 77, 7076.
(c) Su, Z. S.; Li, W. Y.; Wang, J.; Hu, C. W.; Feng, X. M. Chem.- Eur. J. 2013, 19, 1637.
(d) Wang, D.; Liang, J. Y.; Feng, J. C.; Wang, K. R.; Sun, Q. T.; Zhao, L.; Li, D.; Yan, W. J.; Wang, R. Adv. Synth. Catal. 2013, 355, 548.
[37] (a) Vesely, J.; Rios, R. Chem. Soc. Rev. 2014, 43, 611.
(b) Liu, Y. L.; Yin, X. P.; Zhou, J. Chin. J. Chem. 2018, 36, 321.
(c) Iwanejko, J.; Wojaczynska, E. Org. Biomol. Chem. 2018, 16, 7296.
[38] Yan, H. L.; Oh, J. S.; Lee, J. W.; Song, C. E. Nat. Commun. 2012, 3, 2216/1.
[39] (a) Kaur, J.; Chimni, S. S. Org. Biomol. Chem. 2018, 16, 3328.
(b) Wang, H. Y.; Zheng, C.W.; Chai, Z.; Zhang, J. X.; Zhao, G. Nat. Commun. 2016, 7, 12720.
[40] (a) Karahan, S.; Tanyeli, C. Tetrahedron Lett. 2018, 59, 3725.
(b) Zhao, B. L.; Li, J. H.; Du, D. M. Chem. Rec. 2017, 17, 1.
[41] Agnew-Francis, K. A.; Williams, C. M. Adv. Synth. Catal. 2016, 358, 675.
[42] Xue, H. S.; Tan, C. H.; Wong, M. W. Can. J. Chem. 2016, 94, 1099.
[43] Agarwal, J. Org. Biomol. Chem. 2016, 14, 10747.
[44] (a) Hatano, M.; Ishihara, K. Asian J. Org. Chem. 2014, 3, 352.
(b) Ma, J. A.; Zhang, G. W. In Tools for Stereoselective Synthesis, Eds.:Martin, M.; Boysen K., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013, Chapter 16, pp. 351~370.
[45] Hou, Y. L.; Sun, R. W. Y.; Zhou, X. P.; Wang, J. H.; Li, D. Chem. Commun. 2014, 50, 2295.
[46] Mendoza, J. H. Q.; Henao, J. A.; Otero, A. L. C.; Kouznetsov, V. V. Powder Diffr. 2016, 31, 149.
[47] Martinez-Ariza, G.; Nunez-Rios, J.; Lee, Y. S.; Hulme, C. Tetrahedron Lett. 2015, 56, 1038.
[48] Hu, X. C.; Li, R. Z; Li, Z. J. Chem. Res. 2014, 38, 432.
[49] (a) Hall, R. G.; Edmunds, A.; Jeanguenat, A. WO 2014166795, 2014[Chem. Abstr. 2014, 161, 613546].
(b) Buysse, A. M.; Niyaz, N. M.; Demeter, D. A.; Zhang, Y.; Walsh, M. J.; Kubota, A.; Hunter, R.; Trullinger, T. K.; Lowe, C. T.; Knueppel, D.; Patny, A.; Garizi, N.; Leplae, P. R. J.; Wessels, F.; Ross, R. J.; Deamicis, C.; Borromeo, P. US 20140213448, 2014[Chem. Abstr. 2014, 161, 275906].
(c) Dai, H.; Xiao, Y.-S.; Li, Z.; Xu, X.-Y.; Qian, X.-H. Chin. Chem. Lett. 2014, 25, 1014.
[50] (a) Zhang Y. B. World Pestic. 2013, 35, 38(in Chinese). (张一宾, 世界农药, 2013, 35, 38.)
(b) Moffat A. S. Science 1993, 261, 550.
(c) Loso, M. R.; Nugent, B. M.; Huang, J. X.; Rogers, R. B.; Zhu, Y.; Renga, J. M.; Hegde, V. B.; Demark, J. J. WO 2007095229, 2007[Chem. Abstr. 2007, 147, 270793].
(d) Manabe, A.; Enomoto, M.; Yamada, Y.; Oguri, Y.; Sasaki, M. Pestic. Sci. 1999, 55, 649.
[51] (a) Zhang, D. Q.; Xu, G. F.; Liu, Y. H.; Wang, D. Q.; Yang, X. L.; Yuan, D. K. Chin. J. Org. Chem. 2015, 35, 2191(in Chinese). (张大强, 徐高飞, 刘艳红, 王道全, 杨新玲, 袁德凯, 有机化学, 2015, 35, 2191.)
(b) Zhang, D. Q.; Xu, G. F.; Fan, Z. J.; Wang, D. Q.; Yang, X. L.; Yuan, D. K. Chin. Chem. Lett. 2012, 23, 669.
[52] Xu, G. F.; Liu, Y. H.; Yang, X. L.; Wang D. Q.; Yuan, D. K. Chem. J. Chin. Univ. 2016, 37, 486(in Chinese). (徐高飞, 刘艳红, 杨新玲, 王道全, 袁德凯, 高等学校化学学报, 2016, 37, 486.)
/
| 〈 |
|
〉 |