

8-异戊烯基黄酮类天然产物的微波促进Claisen重排合成
收稿日期: 2018-12-11
修回日期: 2019-03-01
网络出版日期: 2019-03-29
基金资助
果蔬加工与质量安全湖南省重点实验室(No.2108TP1030)和湖南省自然科学基金(No.2018JJ2034)资助项目.
Synthesis of 8-Prenylflavonoids Natural Products by Microwave Promoted Claisen Rearrangement
Received date: 2018-12-11
Revised date: 2019-03-01
Online published: 2019-03-29
Supported by
Project supported by the Key Laboratory of Hunan Province for Fruit and Vegetable Processing and Quality Safety (No. 2108TP1030) and the Hunan Provincial Natural Science Foundation of China (No. 2018JJ2034).
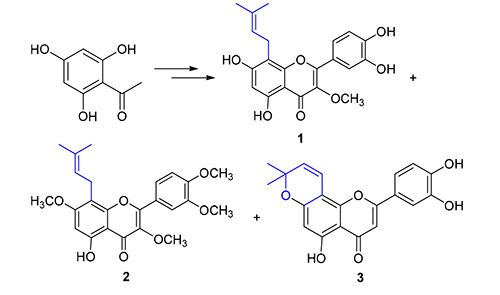
8-异戊烯基黄酮是一类具有显著生物活性的天然产物.以2,4,6-三羟基苯乙酮和3,4-二羟基苯甲醛为原料,用氯甲基甲醚保护羟基,经羟醛缩合、碘催化环合、过氧丙酮(DMDO)氧化、O-异戊烯基化、微波促进的Claisen重排、脱甲氧甲基保护基、O-甲基化和异戊烯基侧链环合等反应步骤,完成了8-异戊烯基槲皮素-3-甲醚(1)、8-异戊烯基槲皮素-3,7,3',4'-四甲醚(2)和Artochamin C(3)这3种8-异戊烯基黄酮类天然产物的合成.并对由微波促进的由5-O-异戊烯基黄酮类化合物合成8-C-异戊烯基黄酮类化合物的Claisen重排反应的关键步骤进行了探讨.所有合成的化合物经1H NMR、13C NMR和MS等结构确证.
李蔚 , 宿亮 , 汪秋安 , 李高阳 , 单杨 . 8-异戊烯基黄酮类天然产物的微波促进Claisen重排合成[J]. 有机化学, 2019 , 39(7) : 1976 -1982 . DOI: 10.6023/cjoc201812020
8-Prenylflavonoids are a class natural products with significant biological activities. The total synthesis of three prenylflavonoid natural products, 8-prenylquercetin-3-methylether (1), 8-prenylquerccetin-3,7,3',4'-tetramethyl ether (2) and Artochamin C (3), was achieved through methoxymethyl protection, aldol condensation, iodine catalytic cyclization, dimethyl sulfoxide (DMSO) oxidation, O-prenylation, microwave promoted Claisen rearrangement, deprotection, O-methylation and prenyl group side chain cyclization, staring from commercially available 2,4,6-trihydroxyacetophenone and 3,4-dihydroxy benzaldehyde. The key step of microwave promoted Claisen rearrangement formed 8-C-prenylflavonoids from 5-O- prenylflavonoids was investigated. All the synthesized compounds were confirmed by 1H NMR、13C NMR and MS techniques.

[1] Dong, X. W.; Liu, Y. J.; Yan, J. Y.; Jiang, C. Y.; Chen, J.; Liu, T.; Hu, Y. Z. Bioorg. Med. Chem. 2008, 16, 8151.
[2] Paoletti, T.; Fallarini, S.; Gugliesi, F.; Minassi, A.; Appendino, G.; Lombardi, G. Eur. J. Pharmacol. 2009, 620, 120.
[3] Daskiewicz, J. B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Barron, D. J. Med. Chem. 2005, 48, 2790.
[4] Shang, M. Y.; Wang, Q. H.; Xiao, J. J. CN 102382092, 2012. Pinhey, J.T.; Southwell, I. A. Austr. J. Chem. 1973, 26, 409.
[5] Wang, Y. H.; Hou, A.J.; Chen, L.; Chen, D. F.; Sun, H. D.; Zhao, Q. S.; Bastow, K. F.; Nakanish, Y.; Wang, X. H.; Lee, K. H. J. Nat. Prod. 2004, 67, 757.
[6] Wang, Z. Q.; Wang, H. H.; Wu, J. Y. Chem. Biol. Interact. 208, 179, 375.
[7] Zeng, L.; Fukai, T.; Nomura, T.; Zhang, R. Y.; Lou, Z. C. J. Chem. Soc., Perkin Trans. 1 1993, 1153.
[8] Majumdar, K. C.; Nandi, R. K. Tetrahedron 2013, 69, 6921.
[9] Daskiewicz, J. B.; Bayet, C.; Barron, D. Tetrahedron Lett. 2001, 42, 7241.
[10] Al-Maharik, N.; Botting, N. P. Tetrahedron 2003, 59, 4177.
[11] Mu, G. M.; Pu, W. C.; Zhou, M.; Liu, Y.; Yang, H. J.; Wang, C. Chin. J. Org. Chem. 2013, 33, 1298(in Chinese). (牟关敏, 蒲文臣, 周敏, 刘燕, 杨海君, 王淳, 有机化学, 2013, 33, 1298.)
[12] Lan, C.; Xu, P.; Liang, R. H.; Xu, Z. L.; Xia, Z. N. Chin. J. Org. Chem. 2012, 32, 765(in Chinese). (兰聪, 徐盼, 梁荣辉, 许泽龙, 夏之宁, 有机化学, 2012, 32, 765.)
[13] Nguyen, V. S.; Dong, L. P.; Wang, S. C.; Wang, Q. A. Eur. J. Org. Chem. 2015, 10, 2297.
[14] Wu, Z.; Cai, S. L.; Fan, W. J.; Wang, Q. A. Chin. J. Org. Chem. 2012, 32, 1296(in Chinese). (吴峥, 蔡双莲, 范文金, 汪秋安, 有机化学, 2012, 32, 1296.)
[15] Detsi, A.; Majdalani, M.; Kontogiorgis, C. A.; Hadjipavlou-Litina, D.; Kefalas, P. Bioorg. Med. Chem. 2009, 17, 8073.
/
| 〈 |
|
〉 |