

4-氨基-5-取代-1,2,4-三唑-3-硫酮席夫碱的合成、生物活性及分子对接
收稿日期: 2018-11-11
修回日期: 2019-01-21
网络出版日期: 2019-04-08
基金资助
陕西省重点自然科学基金(No.2016JZ003)、西北大学研究生自主创新项目(No.YZZ17137)、西北大学大学生创新创业训练计划(No.2018377)资助项目.
Synthesis, Biological Activity and Molecular Docking of 4-Amino-5-substituted-1,2,4-triazole-3-thione Schiff Base
Received date: 2018-11-11
Revised date: 2019-01-21
Online published: 2019-04-08
Supported by
Project supported by the Provincial Key-point Natural Science Foundation of Shaanxi Province (No. 2016JZ003), the Innovation and Creativity Funds for Graduates in Northwest University (No. YZZ17137), the Innovation and Entrepreneurship Training Program for Undergraduates in Northwest University (No. 2018377).
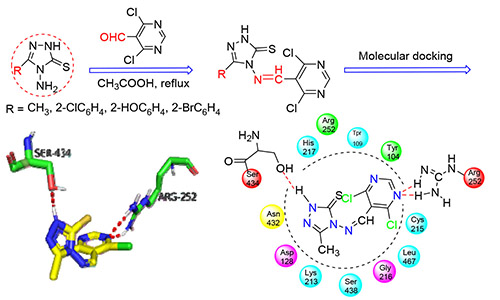
首先分别以硫代甲酰肼与冰乙酸为原料合成了中间体4-氨基-5-甲基-1,2,4-三唑-3-硫酮(M1);以取代苯甲酸为原料经过酯化、酰肼化、成盐和环化合成了中间体4-氨基-5-芳基-1,2,4-三唑-3-硫酮(M2).再将M1和M2分别与4,6-二氯-5-嘧啶甲醛进行加成-消除反应,合成了4种新型含嘧啶环的1,2,4-三唑席夫碱化合物M1-1,M2-1,M2-2和M2-3.通过元素分析、红外光谱分析及1H NMR对其进行结构表征.采用菌丝生长速率法及分子对接法研究目标化合物的生物活性及抑菌机理.结果表明,化合物对不同真菌均有一定的抑制作用.由EC50值可见,化合物M1-1,M2-2和M2-3对小麦赤霉菌的抑菌效果均优于标准药物(氟康唑),且与分子对接结果相一致.
武少婕 , 卢一鸣 , 雷卓楠 , 蒋演 , 张文慧 , 齐乐 , 马海霞 , 任莹辉 . 4-氨基-5-取代-1,2,4-三唑-3-硫酮席夫碱的合成、生物活性及分子对接[J]. 有机化学, 2019 , 39(7) : 1939 -1944 . DOI: 10.6023/cjoc201811016
In this paper, the intermediate of 4-amino-5-methyl-1,2,4-triazole-3-thione (M1) was synthesized with thioformyl hydrazide and acetic acid, and 4-amino-5-aryl-1,2,4-triazole-3-thione (M2) was got by esterification, hydrazide, salt formation and cyclization with substituted benzoic acid. Four compounds of 1,2,4-triazole Schiff bases containing the pyrimidine ring M1-1, M2-1, M2-2 and M2-3 were obtained with 4,6-dichloro-5-pyrimidinecarboxaldehyde and M1/M2 according to the addition-elimination reaction. The structures of the compounds were characterized by elemental analysis, IR and 1H NMR spectra. The bioactivities and antibacterial mechanism of the title compounds were also studied by mycelial growth rate method and molecular docking. The results showed that the compounds had certain inhibitory effects on different fungi. Based on the values of EC50, compounds M1-1, M2-2 and M2-3 have better antifungal effects against the wheat gibberella than that of the standard drug (fluconazole), which is corresponding to the result of molecular docking.

[1] Jin, R. Y. Ph. D. Dissertation, Northwest University, Xi'an, 2015 (in Chinese). (靳如意, 博士论文, 西北大学, 西安, 2015).
[2] Abele, E.; Abele, R.; Lukevics, E. Chem. Heterocycl. Compd. 2008, 44, 769.
[3] Kauthale, S.; Tekale, S.; Damale, M.; Sangshetti, J.; Pawar, R. Bioorg. Med. Chem. Lett. 2017, 27, 3891.
[4] Jiang, S. L.; Han, L. Chin. J. Org. Chem. 2012, 32, 930(in Chinese). (蒋绍亮, 韩亮, 有机化学, 2012, 32, 930.)
[5] Ren, G, Y. Ph. D. Dissertation, Northwest University, Xi'an, 2018 (in Chinese). (任国瑜, 博士论文, 西北大学, 西安, 2018.)
[6] Jin, R. Y.; Zeng, C. Y.; Liang, X. H.; Sun, X. H.; Liu, Y. F.; Wang, Y. Y.; Zhou, S. Bioorg. Chem. 2018, 80, 252.
[7] Sun, X. H.; Tao, Y.; Liu, Y. F.; Jia, Y.Q.; Chen, B. Acta Chim. Sinica 2008, 33, 234(in Chinese). (孙晓红, 陶燕, 刘源发, 贾婴琦, 陈邦, 化学学报, 2008, 33, 234.)
[8] Roman, G. Eur. J. Med. Chem. 2015, 89, 744.
[9] Ünver, Y.; Dügdü, E.; Sancak, K.; Mustafa, E. R.; Karaoglu, S. A. Turk. J. Chem. 2009, 33, 135.
[10] Tehrani, K., Mashayekhi, V.; Azerang, P.; Minaei, S.; Sardari, S.; Kobarfard, F. Ir. J. Pharm. Res. 2015, 14, 63.
[11] Tao, Y.; Sun, X. H.; Liu, Y. F.; Chen, B. Chem. Bioeng. 2016, 33, 50(in Chinese). (陶燕, 孙晓红, 刘源发, 陈邦, 化学与生物工程, 2016, 33, 50.)
[12] Jin, R. Y.; Sun, X. H.; Liu, Y. F.; Ma, H. X. Chemistry 2013, 76, 850(in Chinese). (靳如意, 孙晓红, 刘源发, 马海霞, 化学通报, 2013, 76, 850.)
[13] Zhao, P. L.; Chen, P.; Li, Q.; Hu, M. J.; Diao, P. C.; Pan, E. S.; You, W. W. Bioorg. Med. Chem. Lett. 2016, 26, 3680.
[14] Du, H. T.; Du, H. J. Chin. J. Org. Chem. 2010, 30, 138(in Chinese). (杜海堂, 杜海军, 有机化学, 2010, 30, 138.)
[15] Sahoo, K. P.; Sharma, R.; Pattanayak, P. Med. Chem. Res. 2010, 19, 129.
[16] Bhasin, G.; Srivastava, R.; Singh, R. Org. Prep. Proced. Int. 2017, 49, 371.
[17] Xie, W. L.; Zhang, J. G.; Ma, X. J.; Yang, W. Q.; Zhou, Y.; Tang, X. F.; Zou, Y.; Li, H.; He, J. J.; Xie, S. M.; Zhao, Y. H.; Liu, F. P. Chem. Biol. Drug. Des. 2015; 86, 1088.
[18] Magdy, S.; Omima, M. I. A.; Abdelrhman, E. M.; EI-Shetary, B. A. J. Mol. Struct. 2017, 1145, 330.
[19] Lu, W. T.; Sun, X. H.; Liu, Y. F.; Jin, R. Y. Chemistry 2012, 75, 362(in Chinese). (陆文婷, 孙晓红, 刘源发, 靳如意, 化学通报, 2012, 75, 362)
[20] Tang, G. H.; Zhang, Y.; Zhang Y. P.; Zhou, P. P.; Lin, Z. H.; Wang, Y. Q. Chem. J. Chin. Univ. 2017, 38, 2062(in Chinese). (唐光辉, 张娅, 张玉萍, 周朋朋, 林治华, 王远强, 高等化学学报, 2017, 38, 2062.)
[21] Beteringhe, A.; Racuciu, C.; Balan, C.; Stoican, E.; Patron, L. Adv. Mater. Res. 2013, 787, 236.
[22] El Bindary, A. A.; Shoair, A. F.; El-Sonbati, A. Z.; Diab, M. A.; Abdo, E. E. J. Mol. Liq. 2015, 212, 579.
[23] Wu, A. B. Ph.D. Dissertation, Huazhong Agricultural University, Wuhan, 2005 (in Chinese). (武爱波, 博士论文, 华中农业大学, 武汉, 2005.)
[24] Park, Y.; Cho, Y.; Lee, Y. H.; Lee, Y. W.; Rhee, S. J. Struct. Biol. 2016, 194, 395.
[25] Abd-Rabou, A. A.; Abdel-Wahab, B. F.; Bekheit, M. S. Chem. Pap. 2018, 72, 2231.
[26] El Ashry El Sayed H. El Ashry.; El Sayed H. El Tamany.; Mohy El Din Abd El Fattah.; Ahmed T. A. Boraei.; Heba M. Abd El-Nabi. Eu. J. Med. Chem. 2013, 66, 112.
[27] Zhou, J. H.; Li, J. J.; Li, J.; Ren, G. Y.; Ren, R. Y.; Ma, H. X. Chem. Res. Chin. Univ. 2017, 33, 866.
/
| 〈 |
|
〉 |