

氯胺-T促进的醛、肼与富马酸酯的三组分反应构建四取代吡唑类化合物
收稿日期: 2019-01-10
修回日期: 2019-03-18
网络出版日期: 2019-04-09
基金资助
上海市自然科学基金(No.15ZR1401400)资助项目.
Chloroamine-T Promoted Three-Component Reaction of Aldehydes, Hydrazines and Fumarate Esters for the Construction of Tetra-substituted Pyrazole Derivatives
Received date: 2019-01-10
Revised date: 2019-03-18
Online published: 2019-04-09
Supported by
Project supported by the Natural Science Foundation of Shanghai City (No. 15ZR1401400).
陈樱 , 祝家楠 , 赵圣印 . 氯胺-T促进的醛、肼与富马酸酯的三组分反应构建四取代吡唑类化合物[J]. 有机化学, 2019 , 39(7) : 1923 -1929 . DOI: 10.6023/cjoc201901014
Pyrazoles, a class of five-membered nitrogen-containing heterocyclic compounds, showed antibacterial, anticancer and antioxidative activity, and so on. They also served as important intermediates in organic synthesis. To develop the general and straightforward methods to their synthesis is of great significance. In this paper, a series of tetra-substituted pyrazoles were synthesized from aldehydes, hydrazines and fumarate esters by three-component[3+2] cycloaddition reaction in the presence of chloroamine-T with 60%~87% yields. Their structures were confirmed by 1H NMR, 13C NMR, IR and HRMS analysis.
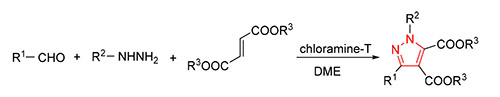
Key words: aldehyde; aldehyde; fumarate ester; chloroamine-T; pyrazole
[1] For biological reviews:(a) Silva, V. L. M.; Elguero, J.; Silva, J.M. S. Eur. J. Med. Chem. 2018, 156, 394.
(b) Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y. N.; Al-aizari, F. A.; Ansar, M. Molecules 2018, 23, 134/1.
(c) Faria, J. V.; Vegi, P. F.; Miguita, A. G. C.; dos Santos, M. S.; Boechat, N.; Bernardino, A.M. R. Bioorg. Med. Chem. 2017, 25, 5891.
(d) Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman New J. Chem. 2017, 41, 16.
(e) Gangulv, S.; Jacob, S. K. Mini-Rev. Med. Chem. 2017, 17, 959.
[2] For synthetic reviews:(a) Liu, S. Y.; Bao, X. Z.; Wang, B. M. Chem. Commun. 2018, 54, 11515.
(b) Maddila, S.; Jonnalagadda, S. B.; Gangu, K. K.; Maddila, S. N. Curr. Org. Synth. 2017, 14, 634.
(c) Khidre, R. E.; Abdel-Wahab, B. F.; Farahat, A. A.; Mohamed, H. A. J. Heterocycl. Chem. 2016, 53, 13.
(d) Li, M.; Zhao, B. X. Eur. J. Med. Chem. 2014, 85, 311.
[3] (a) Kumar, V.; Kaur, K.; Gupta, G. K.; Sharma, A. K. Eur. J. Med. Chem. 2013, 69, 735.
(b) Schmidt, A.; Dreger, A. Curr. Org. Chem. 2011, 15, 1423.
[4] (a) Domling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083.
(b) Wan, J.-P.; Liu, Y.-Y. RSC Adv. 2012, 2, 9763.
(c) Liu, Y.-Y.; Wang, H.; Wan, J.-P. Asian J. Org. Chem. 2013, 2, 374.
(d) Wan, J.-P.; Gan, L.; Liu, Y.-Y. Org. Biomol. Chem. 2017, 15, 9031.
(e) Rotstein, B.H..; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323.
[5] (a) Wan, J.-P.; Cao, S.; Liu, Y.-Y. J. Org. Chem. 2015, 80, 9028.
(b) Li, Y.; Wei, L.; Wan, J.-P.; Wen, C.-P. Tetrahedron 2017, 73, 2323.
(c) Li, Y.-F.; Huang, J.-W.; Gu, J.-C.; Huang, H.; Niu, C.-S.; Ma, H.-F. Chin. J. Org. Chem. 2016, 36, 520(in Chinese). (李玉峰, 黄佳维, 顾嘉超, 黄浩, 钮长盛, 马鸿飞, 有机化学, 2016, 36, 520.)
(d) Zhou, K. D.; Xia, H.-G.; Wu, J. Org. Chem. Front. 2016, 3, 865.
(e) Wagner, B.; Hiller, W.; Ohno, H.; Krause, N. Org. Biomol. Chem. 2016, 14, 1579.
(f) Xu P.; Li W.; Xie J.; Zhu C. Acc. Chem. Res. 2018, 51, 484.
(g) Pozgan, F.; AlMamari, H.; Groselj, U.; Syete, J.; Stefane, B. Molecules 2018, 23, 3/1.
(h) Liu, K.; Shang, X. Y.; Cheng, Y. Y.; Chang, X. Y.; Li, P. F.; Li, W. J. Org. Biomol. Chem. 2018, 16, 7811.
[6] Yavari, I.; Khalili, G.; Mirzaei, A. Helv. Chim. Acta 2010, 93, 277.
[7] Ma, C.; Li, Y.; Wen, P.; Yan, R.; Ren, Z.; Huang, G. Synlett 2011, 1321.
[8] Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A. R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Adv. Synth. Catal. 2012, 354, 3095.
[9] Rai, L.K.M.; Hassner, A. Synth. Commun. 1989, 19, 2799
[10] Jedlovska, E.; Fisera, L.; Liptaj, T. Chem. Pap. 2005, 59, 354.
[11] Kumar, G. V.; Khatoon, B. B. A.; Kumar, K. A. J. Chem. Pharm. Res. 2015, 7, 854.
[12] Zhu, J. N.; Yang, Z. H.; Qi, M.; Zhao, S. Y. Adv. Synth. Catal. 2019, 361, 868.
[13] Liu, G. N.; Luo, R. H.; Zhou, Y.; Zhang, X. J.; Li, J.; Yang, L. M.; Zheng, Y. T.; Liu, H. Molecules 2016, 21, 1198.
[14] Pratapan, S.; Scaria, P. M.; Bhattacharyya, K.; Das, P. K.; George, M. V. J. Org. Chem. 1986, 51, 1972
[15] Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A. R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. New J. Chem. 2013, 37, 2037.
[16] Mertzanos, G. E.; Alexandrou, N. E.; Tsoleridis, C. A.; Mitkidou, S.; Stephanidou-Stephanatou, J. Heterocycles 1994, 37, 967.
[17] Tewari, R. S.; Parihar, P. Tetrahedron 1983, 39, 129.
[18] Jones, R. G.; Whitehead, C. W. J. Org. Chem. 1955, 20, 1342.
/
| 〈 |
|
〉 |