

交联壳聚糖微球负载铜水相催化Ullmann反应
收稿日期: 2019-01-14
修回日期: 2019-03-25
网络出版日期: 2019-04-11
基金资助
国家自然科学基金(No.21476194)、国家重点研发计划(No.2016YFB0301800)资助项目.
Cross-Linked Chitosan Bead Supported Copper Complex in Water as a Green and Efficient Catalytic Protocol for Ullmann Reaction
Received date: 2019-01-14
Revised date: 2019-03-25
Online published: 2019-04-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21476194), and the National Key Research and Development Program of China (No. 2016YFB0301800).
吕小妹, 阮建成, 陈新志, 钱超 . 交联壳聚糖微球负载铜水相催化Ullmann反应[J]. 有机化学, 2019 , 39(6) : 1720 -1726 . DOI: 10.6023/cjoc201901018
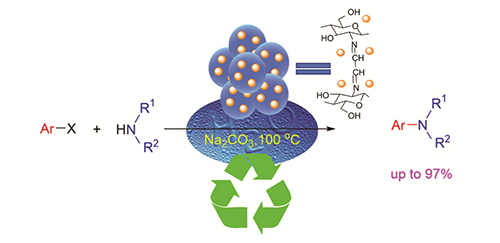
A green, efficient, and recyclable catalytic protocol for Ullmann C-N reaction in water was developed. The catalyst Chi-Gly@CuI was prepared by the cross-linking reaction of chitosan bead with glyoxal and subsequently anchored with copper salt. Chi-Gly@CuI bead of 0.3 mm in mean diameter possesses porous micro-structure demonstrated by scanning electron microscope (SEM). The structure of Chi-Gly@CuI was characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TG), X-ray diffraction (XRD), inductively coupled plasma-atomic emission spectrometry (ICP-AES), and X-ray photoelectron spectroscopy (XPS). This catalytic protocol for Ullmann reaction in water exhibited high applicability, from which the corresponding coupling products were afforded in good to excellent yields. Chi-Gly@CuI could be easily separated from products by simple filtration almost without weight loss. Most notably, after 10 times of recycling, its catalytic activity and chemical stability were still maintained.
[1] Ferlin, F.; Trombettoni, V.; Luciani, L.; Fusi, S.; Piermatti, O.; Santoro, S.; Vaccaro, L. Green Chem. 2018, 20, 1634.
[2] Bariwal, J.; Van der Eycken, E. Chem. Soc. Rev. 2013, 42, 9283.
[3] Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 6954.
[4] Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem Rev 2002, 102, 1359.
[5] Beletskaya, I. P.; Cheprakov, A. V. Coord. Chem. Rev. 2004, 248, 2337.
[6] Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Chem. Soc. Rev. 2014, 43, 3525.
[7] Monnier, F.; Taillefer, M., Angew. Chem., Int. Ed. 2008, 47, 3096.
[8] Hartwig, J. F. Angew. Chem., Int. Ed. 1998, 37, 2046.
[9] Klapars, A.; Antilla, J. C.; Huang, X. H.; Buchwald, S. L. J. Am. Chem. Soc. 2001, 123, 7727.
[10] Job, G. E.; Buchwald, L. Org. Lett. 2002, 4, 3703.
[11] Klapars, A.; Huang, X. H.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 7421.
[12] Ma, D. W.; Cai, Q. Synlett 2004, (1), 128.
[13] Cheng, D. P.; Gan, F. F.; Qian, W. X.; Bao, W. L. Green Chem. 2008, 10, 171.
[14] Kwong, F. Y.; Klapars, A.; Buchwald, S. L. Org. Lett. 2002, 4, 581.
[15] Kwong, F. Y.; Buchwald, S. L. Org. Lett. 2003, 5, 793.
[16] Antilla, J. C.; Baskin, J. M.; Barder, T. E.; Buchwald, S. L. J. Org. Chem. 2004, 69, 5578.
[17] Cristau, H. J.; Cellier, P. P.; Spindler, J. F.; Taillefer, M. Chem.-Eur. J. 2004, 10, 5607.
[18] Choudary, B. M.; Sridhar, C.; Kantam, M. L.; Venkanna, G. T.; Sreedhar, B. J. Am. Chem. Soc. 2005, 127, 9948.
[19] Strieter, E. R.; Blackmond, D. G.; Buchwald, S. L. J. Am. Chem. Soc. 2005, 127, 4120.
[20] Zhang, H.; Cai, Q.; Ma, D. W. J. Org. Chem. 2005, 70, 5164.
[21] Zhu, W.; Ma, D. W. J. Org. Chem. 2005, 70, 2696.
[22] Shafir, A.; Buchwald, S. L. J. Am. Chem. Soc. 2006, 128, 8742.
[23] Ma, D. W.; Cai, Q. A. Acc. Chem. Res. 2008, 41, 1450.
[24] Cassez, A.; Ponchel, A.; Hapiot, F.; Monflier, E. Org. Lett. 2006, 8, 4823.
[25] Xue, C. H.; Palaniappan, K.; Arumugam, G.; Hackney, S. A.; Liu, J.; Liu, H. Y. Catal. Lett. 2007, 116, 94.
[26] Ngah, W. S. W.; Fatinathan, S. Chem. Eng. J. 2008, 143, 62.
[27] Baig, R. B. N.; Varma, R. S. Green Chem. 2013, 15, 1839.
[28] Dekamin, M. G.; Azimoshan, M.; Ramezani, L. Green Chem. 2013, 15, 811.
[29] Zhang, G. F.; Luan, Y. X.; Han, X. W.; Wang, Y.; Wen, X.; Ding, C. R.; Gao, J. R. Green Chem. 2013, 15, 2081.
[30] Baig, R. B. N.; Nadagouda, M. N.; Varma, R. S. Green Chem. 2014, 16, 2122.
[31] Shen, C.; Xu, J.; Yu, W. B.; Zhang, P. F. Green Chem. 2014, 16, 3007.
[32] Yang, B.; Mao, Z. X.; Zhu, X. H.; Wan, Y. Q. Catal. Commun. 2015, 60, 92.
[33] Cui, Q.; Zhao, H.; Luo, G.; Xu, J. Ind. Eng. Chem. Res. 2017, 56, 143.
[34] Ma, L.; Su, Y.; Chen, J.; Xu, J. Ind. Eng. Chem. Res. 2017, 56, 12655.
[35] Su, Y.; Ma, L.; Chen, J.; Xu, J. Carbohydr. Polym. 2017, 175, 113.
[36] Tong, J. H.; Zhen, L.; Xia, C. G. J. Mol. Catal. A 2005, 231(1-2), 197.
[37] Anuradha; Kumari, S.; Pathak, D. D. Tetrahedron Lett. 2015, 56, 4135.
[38] Keshipour, S.; Mirmasoudi, S. S. Carbohydr. Polym. 2018, 196, 494.
[39] Gioia, C.; Ricci, A.; Bernardi, L.; Bourahla, K.; Tanchoux, N.; Robitzer, M.; Quignard, F. Eur. J. Org. Chem. 2013, (3), 588.
[40] Chanda, A.; Fokin, V. V. Chem. Rev. 2009, 109, 725.
[41] Krause, N. Curr. Opin. Green Sustainable Chem. 2017, 7, 18.
[42] La Sorella, G.; Strukul, G.; Scarso, A. Green Chem 2015, 17, 644.
[43] Lipshutz, B. H.; Ghorai, S.; Cortes-Clerget, M. Chemistry 2018, 24, 6672.
[44] Rani, D.; Singla, P.; Agarwal, J. Carbohydr. Polym. 2018, 202, 355.
[45] Ge, X.; Qian, C.; Chen, X. Z. Tetrahedron:Asymmetry 2014, 25, 1450.
[46] Ge, X.; Qian, C.; Chen, Y. B.; Chen, X. Z. Tetrahedron:Asymmetry 2014, 25, 596.
[47] Ge, X.; Chen, X. Z.; Qian, C.; Zhou, S. D. RSC Adv. 2016, 6, 58898.
[48] Ge, X.; Chen, X. Z.; Qian, C.; Zhou, S. D. RSC Adv. 2016, 6, 29638.
[49] Ge, X.; Sun, F.; Liu, X.; Chen, X.; Qian, C.; Zhou, S. New J. Chem. 2017, 41, 13175.
[50] Liu, X.; Chang, S.; Chen, X.; Ge, X.; Qian, C. New J. Chem. 2018, 42, 16013.
[51] Sowmya, A.; Meenakshi, S. Int. J. Biol. Macromol. 2014, 64, 224.
[52] Saha, S.; Das, S.; Sen, D.; Ghorai, U. K.; Mazumder, N.; Gupta, B. K.; Chattopadhyay, K. K. J. Mater. Chem. C 2015, 3, 6786.
[53] Kumar, M.; Bhatt, V.; Nayal, O. S.; Sharma, S.; Kumar, V.; Thakur, M. S.; Kumar, N.; Bal, R.; Singh, B.; Sharma, U. Catal. Sci. Technol. 2017, 7, 2857.
[54] Zeng, L. X.; Chen, Y. F.; Zhang, Q. Y.; Guo, X. M.; Peng, Y. N.; Xiao, H. J.; Chen, X. C.; Luo, J. W. Carbohydr. Polym. 2015, 130, 333.
[55] Zhou, Y. T.; Branford-White, C.; Nie, H. L.; Zhu, L. M. Colloid Surface B 2009, 74, 244.
/
| 〈 |
|
〉 |