

L-鸟嘌呤异核苷的全合成研究
收稿日期: 2019-01-27
修回日期: 2019-03-18
网络出版日期: 2019-04-16
基金资助
国家自然科学基金(Nos.21462019,21676131)、江西省科技厅重点(No.20143ACB20012)和江西科技师范大学博士启动基金(No.2018BSQD022)资助项目.
Studies on the Total Synthesis of iso-L-Guanosine
Received date: 2019-01-27
Revised date: 2019-03-18
Online published: 2019-04-16
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21462019, 21676131), the Bureau of Science & Technology of Jiangxi Province (No. 20143ACB20012) and the Jiangxi Science & Technology Normal University (Doctor Startup Fund No. 2018BSQD022).
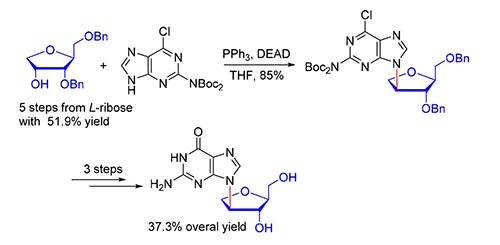
发展了一条改进的L-鸟嘌呤异核苷全合成路线.以L-核糖为起始原料,合成了3,5-O-二苄基-1-脱氧-L-核糖,再与碱基N2,N2-二叔丁氧羰基-6-氯鸟嘌呤发生关键的Mitsunobu反应来合成异核苷6.经过9步反应,以37.3%的总收率合成了L-鸟嘌呤异核苷,其中Mitsunobu反应构建异核苷键具有立体专一性、高产率、条件温和、区域选择性高等优点.该方法可以作为鸟嘌呤异核苷的通用合成路线.
关键词: 核苷; 全合成; 糖基化; Mitsunobu反应; 异核苷
唐杰 , 董祥有 , 欧阳文良 , 朱云龙 , 丁海新 , 肖强 . L-鸟嘌呤异核苷的全合成研究[J]. 有机化学, 2019 , 39(9) : 2609 -2615 . DOI: 10.6023/cjoc201901045
An improved route for the total synthesis of iso-L-guanosine was developed. Using L-ribose as the starting material, 3,5-O-dibenzyl-1-deoxy-L-ribose was firstly synthesized. Then, Mitsunobu reaction between N2,N2-bis(tert-butyloxycarbonyl)-6-chloro-guanine and 3,5-O-dibenzyl-1-deoxy-L-ribose afforded isonucleoside 6. Finally, iso-L-guanosine was synthesized in 9 steps with 37.3% overall yield. Adopting Mitsunobu reaction as the key step, it has the merits of high steroseletivity and regioselectivity, mild reaction condition, and high yield. Currently developed approach could be used as a general synthetic strategy for the synthesis other related guanine isonucleosides.

Key words: nucleosides; total synthesis; glycosidation; Mitsunobu reaction; isonucleosides
[1] (a) Yates, M. K.; Seley-Radtke, K. L. Antiviral Res. 2019, 162, 5.
(b) Seley-Radtke, K. L.; Yates, M. K. Antiviral Res. 2018, 154, 66.
(c) Jordheim, L. P.; Durantel, D.; Zoulim, F.; Dumontet, C. Nat. Rev. Drug Discovery 2013, 12, 447.
(d) Zhou, X.-X.; Littler, E. Curr. Top. Med. Chem. 2006, 6, 851.
(e) Fan, X.-S.; Zhang, X.-Y.; Wang, X.; Qu, G.-R. Chin. J. Org. Chem. 2008, 28, 1888(in Chinese). (范学森, 张新迎, 王霞, 渠桂荣, 有机化学, 2008, 28, 1888.)
[2] (a) Montgomery, J. A.; Clayton, S. D.; Thomas, H. J. J. Org. Chem. 1975, 40, 1923.
(b) Montgomery, J. A.; Thomas, H. J. J. Org. Chem. 1978, 43, 541.
[3] (a) Nair, V.; Jahnke, T. S. Antimicrob. Agents Chemother. 1995, 39, 1017.
(b) Nair, V.; Piotrowska, D. G.; Okello, M.; Vadakkan, J. Nucleosides Nucleotides Nucleic Acids 2007, 26, 687.
(c) Chun, B. K.; Vadakkan, J. J.; Nair, V. Nucleosides Nucleotides Nucleic Acids 2005, 24, 725.
[4] (a) Ogino, T.; Sato, K.; Matsuda, A. ChemBioChem 2010, 11, 2597.
(b) Kira, T.; Kakefuda, A.; Shuto, S.; Matsuda, A.; Baba, M.; Shigeta, S. Nucleosides Nucleotides Nucleic Acids 1995, 14, 571.
(c)Yoshimura, Y.; Asami, K.; Matsui, H.; Tanaka, H.; Takahata, H. Org. Lett. 2006, 8, 6015.
[5] (a) Yu, H. W.; Zhang, H. Y.; Yang, Z. J.; Min, J. M.; Ma, L. T.; Zhang, L. H. Pure App. Chem. 1998, 70, 435.
(b) Tian, X. B.; Min, J. M.; Zhang, L. H. Tetrahedron:Asymmetry 2000, 11, 1877.
(c) Yu, H. W.; Zhang, L. R.; Zhou, J. C.; Ma, L. T.; Zhang, L. H. Bioorg. Med. Chem. 1996, 4, 609.
[6] (a) Song, Y.; Yang, R.; Ding, H.; Sun, Q.; Xiao, Q.; Ju, Y. Synthesis-Stuttgart 2011, 1213.
(b) Sun, Z. D.; Zhu, Y. L.; Huang, H. Y.; Song, X. R.; Xiao, Q. Chin. J. Org. Chem. 2016, 36, 2729(in Chinsese). (孙志东, 朱云龙, 黄海洋, 宋贤荣, 肖强, 有机化学, 2016, 36, 2729.)
[7] Huang, Y.; Chen, Z.; Chen, Y.; Zhang, H.; Zhang, Y.; Zhao, Y.; Yang, Z.; Zhang, L. Bioconjugate Chem. 2013, 24, 951.
[8] Cai, B.; Yang, X.; Sun, L.; Fan, X.; Li, L.; Jin, H.; Wu, Y.; Guan, Z.; Zhang, L.; Zhang, L.; Yang, Z. Org. Biomol. Chem. 2014, 12, 8866.
[9] Fan, X.; Sun, L.; Li, K.; Yang, X.; Cai, B.; Zhang, Y.; Zhu, Y.; Ma, Y.; Guan, Z.; Wu, Y.; Zhang, L.; Yang, Z. Mol. Ther.-Nucleic Acids 2017, 9, 218.
[10] Li, L.; Yang, X.; Li, K.; Zhang, G.; Ma, Y.; Cai, B.; Li, S.; Ding, H.; Deng, J.; Nan, X.; Sun, J.; Wu, Y.; Shao, N.; Zhang, L.; Yang, Z. Org. Biomol. Chem. 2018, 16, 7488.
[11] (a) Zhang, S.; Cao, M.; Guan, Z.; Wang, Z.; Cao, Y. L.; Guo, Y.; Yang, Z. J.; Zhang, L. H. Chin. J. Med. Chem. 2011, 2, 4.
(b) Zhang, S. M.S. Thesis, Peking University, Beijing, 2011(in Chinese). (张烁, 硕士论文, 北京大学, 北京, 2011.)
(c) Zhang, H. Y.; Wu, X. J.; Yu, H. W.; Ma, L. T.; Zhang, L. H. Chin. Chem. Lett. 1996, 7, 1089.
(d) Zhang, H. Y.; Zhang, M. L.; Piao, Z. S.; Ma, L. T.; Zhang, L. H. Acta Pharm. Sin. 1999, 34, 363(in Chinese). (张虎翼, 张铭龙, 朴志松, 马灵台, 张礼和, 药学学报, 1999, 34, 363.)
[12] (a) Zhang, P. S.; Dong, E Z. M.; Cleary, T. P. Org. Process Res. Dev. 2005, 9, 583.
(b) Forsman, J. J.; Waerna, J.; Murzin, D. Y.; Leino, R. Eur. J. Org. Chem. 2009, 5666.
[13] CCDC 1892867(Compound 8) contain the supplementary crystallographic data for this paper.
[14] Houston, T. A.; Koreeda, M. Carbohydr. Res. 2009, 344, 2240.
[15] Kakefuda, A.; Shuto, S.; Nagahata, T.; Seki, J.; Sasaki, T.; Matsuda, A. Tetrahedron 1994, 50, 10167.
[16] Ohrui, H.; Waga, T.; Meguro, H. Biosci. Biotechnol. Biochem. 1993, 57, 1040.
[17] (a) Yoshimura, Y. Heterocycles 2017, 94, 1625.
(b) Kitkowska, J. D.; Tabaczynska, Z. A.; Draminski, M. Wiad. Chem. 2013, 67, 843.
(c) Leclerc, E. In Chemical Synthesis of Carbocyclic Analogues of Nucleosides, John Wiley & Sons, Inc., New York, 2013, pp. 535~604.
[18] (a) Jacobsen, M. F.; Knudsen, M. M.; Gothelf, K. V. J. Org. Chem. 2006, 71, 9183.
(b) Mercurio, M. E.; Tomassi, S.; Gaglione, M.; Russo, R.; Chambery, A.; Lama, S.; Stiuso, P.; Cosconati, S.; Novellino, E.; Di Maro, S.; Messere, A. J. Org. Chem. 2016, 81, 11612.
(c) Zhou, J.; Du, X.; Chen, X.; Xu, B. Biochemistry 2018, 57, 4867.
(d) Porcheddu, A.; Giacomelli, G.; Piredda, I.; Carta, M.; Nieddu, G. Eur. J. Org. Chem. 2008, (34), 5786.
[19] (a) Tzeng, C.-C.; Hwang, L.-C.; Chen, C.-C.; Wei, D.-C. Nucleoside Nucleotides 1995, 14, 1425.
[20] Meade, E. A.; Wotring, L. L.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 1997, 40, 794.
[21] Lenagh-Snow, G. M. J.; Araujo, N.; Jenkinson, S. F.; Rutherford, C.; Nakagawa, S.; Kato, A.; Yu, C.-Y.; Weymouth-Wilson, A. C.; Fleet, G. W. J. Org. Lett. 2011, 13, 5834.
/
| 〈 |
|
〉 |