

芳甲基叠氮化合物与烯烃及其衍生物反应研究进展
收稿日期: 2019-03-01
修回日期: 2019-03-30
网络出版日期: 2019-04-16
基金资助
国家自然科学基金(No.21761033)、广西自然科学基金(Nos.2017GXNSFBA198211,2018GXNSFAA294064)以及玉林师范学院科研(Nos.2018YJKY36,201810606010)项目资助.
Recent Progress on Reactions of Arylmethyl Azides with Alkenes
Received date: 2019-03-01
Revised date: 2019-03-30
Online published: 2019-04-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21761033), the Natural Science Foundation of Guangxi (Nos. 2017GXNSFBA198211, 2018GXNSFAA294064), and the Yulin Normal University Research (Nos. 2018YJKY36, 201810606010).
李秀英 , 李亚军 , 韦贤生 , 罗金荣 , 黄国保 , 谭明雄 . 芳甲基叠氮化合物与烯烃及其衍生物反应研究进展[J]. 有机化学, 2019 , 39(7) : 1831 -1836 . DOI: 10.6023/cjoc201903001
Arylmethyl azides (ArCH2N3) as one of the significant nitrogen sources with stable properties, simple synthesis, have been widely used in a wide range of organic synthesis reactions. The recent progress (2014~2018) on reactions of arylmethyl azides with alkenes is summarized. In addition, the organic reactions of arylmethyl azides with types of alkenes are described respectively, with their scope of substrates and reaction mechanism. It is hoped that this review can be referred to the future application in organic synthesis of arylmethyl azides with alkenes.
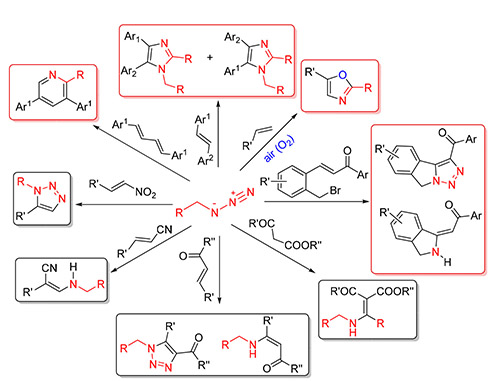
Key words: arylmethyl azides; alkenes; reaction mechanism
[1] Grecianl, S.; Aubé, J. Organic Azides:Syntheses and Applications, Vol. 7, Eds.:Bräse, S.; Banert, K., John Wiley & Sons, Ltd, Chichester, UK, 2010, pp. 191~310.
[2] (a) Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Angew. Chem., Int. Ed. 2005, 44, 5188.
(b) Lee, J. H.; Gupta, S.; Jeong, W.; Rhee, Y. H.; Park, J. Angew. Chem., Int. Ed. 2012, 51, 10851.
(c) Han, J.; Jeon, M.; Pak, H. K.; Rhee, Y. H.; Park, J. Adv. Synth. Catal. 2014, 356, 2769.
(d) Gupta, S.; Han, J.; Kim, Y.; Lee, S. W.; Rhee, Y. H.; Park. J. J. Org. Chem. 2014, 79, 9094.
(e) Chou, H.-H.; Raines, R. T. J. Am. Chem. Soc. 2013, 135, 14936.
(f) Zhang, X.-X.; Sun, X.-P.; Zhang, H.-F.; Cui, X.-L.; Ma, M.-T. Chin. J. Org. Chem. 2015, 35, 1469(in Chinese). (张小祥, 孙小萍, 张海飞, 崔杏丽, 马猛涛, 有机化学, 2015, 35, 1469.)
(g) Zhang, W.-S.; Xu, W.-J.; Kuang, C.-X. Chin. J. Org. Chem. 2015, 35, 2059(in Chinese). (张文生, 许文静, 匡春香, 有机化学, 2015, 35, 2059.)
[3] (a) Song, Z.-Q.; Zhao, Y.-M.; Zhai, H.-B. Org. Lett. 2011, 13, 6331.
(b) Lamani, M.; Devadig, P.; Prabhu, K. R. Org. Biomol. Chem. 2012, 10, 2753.
(c) Tummatorn, J.; Thongsornkleeb, C.; Ruchirawat, S.; Gettongsong, T. Org. Biomol. Chem. 2013, 11, 1463.
[4] Shin, K.; Kim, H.; Chang, S. Acc. Chem. Res. 2015, 48, 1040.
[5] Li, J.-L.; Wang, Y.-C.; Li, W.-Z.; Wang, H.-S.; Mo, D.-L.; Pan, Y.-M. Chem. Commun. 2015, 51, 17772.
[6] Tummatorn, J.; Poonsilp, P.; Nimnual, P.; Janprasit, J.; Thongsornkleeb, C.; Ruchirawat, S. J. Org. Chem. 2015, 80, 4516.
[7] Wang, Y.-C.; Li, J.-L.; He, Y.; Xie, Y.-Y.; Wang, H.-S.; Pan, Y.-M. Adv. Synth. Catal. 2015, 357, 3229.
[8] Wang, Y.-C.; Xie, Y.-Y.; Qu, H.-E.; Wang, H.-S.; Pan, Y.-M.; Huang, F. -P. J. Org. Chem. 2014, 79, 4463.
[9] Zefirov, N. S.; Chapovskaya, N. K.; Kolesnikov, V. V. Chem. Commun. 1971, 1001.
[10] Piet, J. C.; Le H. G.; Cailleux, P.; Benhaoua, H.; Carrie, R. Bull. Soc. Chim. Belg. 1996, 105, 33.
[11] Amantini, D.; Fringuelli, F.; Piermatti, O.; Pizzo, F.; Zunino, E.; Vaccaro, L. J. Org. Chem. 2005, 70, 6526.
[12] Wang, Y.-C.; Xie, Y.-Y.; Tan, X.-C.; Wang, H.-S.; Pan, Y.-M. Org. Biomol. Chem. 2015, 13, 513.
[13] Donald, A. S. R.; Marks, R. E. J. Chem. Soc. C 1967, 1188.
[14] Casey, M.; Donnelly, J. A.; Ryan, J. C.; Ushioda, S. ARKIVOC 2003, 7, 310.
[15] (a) Reddy, D. S.; Judd, W. R.; Aubé, J. Org. Lett. 2003, 5, 3899.
(b) Silvio, C.; Amenson, T. G. Tetrahedron Lett. 2012, 53, 6710.
[16] Mahoney, J. M.; Smith, C. R.; Johnston, J. N. J. Am. Chem. Soc. 2005, 127, 1354.
[17] Jumreang, T.; Charnsak, T.; Somsak, R.; Tanita, G. Org. Biomol. Chem. 2013, 11, 1463.
[18] Xie, Y.-Y.; Wang,Y.-C.; Qu, H.-E.; Tan, X.-C.; Wang, H.-S.; Pan, Y.-M. Adv. Synth. Catal. 2014, 356, 3347.
[19] Gangaprasad, D.; Paul Raj, J.; Kiranmye, T.; Sagubar Sadik, S.; Elangovan, J. RSC Adv. 2015, 5, 63473.
[20] Yang, W.-C.; Miao, T.; Li, P.-H.; Wang, L. RSC Adv. 2015, 5, 95833.
[21] Gangaprasad, D.; Paul Raj, J.; Kiranmye, T.; Karthikeyan, K.; Elangovan, J. Eur. J. Org. Chem. 2016, 5642.
[22] Xie, Y.-Y.; Wang, Y.-C.; He, Y.; Hu, D.-C.; Wang, H.-S.; Pan, Y.-M. Green Chem. 2017, 19, 656.
[23] Yan, Z.-M.; Wu, N.; Liang, D.; Wang, H.-S.; Pan, Y.-M. Org. Lett. 2014, 16, 4048.
/
| 〈 |
|
〉 |