

钴催化芳香族烯烃的脱氢硅化反应
收稿日期: 2019-03-10
修回日期: 2019-04-03
网络出版日期: 2019-04-16
基金资助
国家自然科学基金(No.21772171)、国家重点基础研究发展计划(No.2015CB856600)、浙江省自然科学基金(No.LR19B020001)、浙江大学曹光彪高科技发展基金和浙江大学基本科研业务费资助项目.
Cobalt-Catalyzed Dehydrogenative Silylation of Vinylarenes
Received date: 2019-03-10
Revised date: 2019-04-03
Online published: 2019-04-16
Supported by
Project supported by the National Natural Science Foundation of China (No. 21772171), the National Basic Research Program of China (973 Program, No. 2015CB856600), the Zhejiang Provincial Natural Science Foundation (No. LR19B020001), the K. P. Chao's High Technology Development Foundation of Zhejiang University and the Fundamental Research Funds for the Central Universities.
程彪 , 陆鹏 , 赵家金 , 陆展 . 钴催化芳香族烯烃的脱氢硅化反应[J]. 有机化学, 2019 , 39(6) : 1704 -1710 . DOI: 10.6023/cjoc201903018
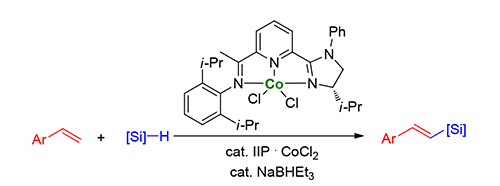
A highly chemo-, regio-, and stereo-selective cobalt-catalyzed dehydrogenative silylation of vinylarenes was described. The imidazoline iminopyridine cobalt complex could promote this reaction effectively and improve the chemoselectivity dramatically. This protocol used earth-abundant transition metal, readily available alkenes and hydrosilanes to construct valuable vinylsilanes. The reaction could be carried out in gramscale and the proposed mechanism was also described.
[1] (a) Chan, T. H.; Fleming, I. Synthesis 1979, 761.
(b) Blumenkopf, T. A.; Overman, L. E. Chem. Rev. 1986, 86, 857.
(c) Langkopf, E.; Schinzer, D. Chem. Rev. 1995, 95, 1375.
(d) Fleming, I.; Barbero, A.; Water, D. Chem. Rev. 1997, 97, 2063.
[2] (a) McAtee, J. R.; Martin, S. E. S.; Ahneman, D. T.; Johnson, K. A.; Watson, D. A. Angew. Chem., Int. Ed. 2012, 51, 3663.
(b) Martin, S. E. S.; Watson, D. A. J. Am. Chem. Soc. 2013, 135, 13330.
[3] (a) Na, Y.; Chang, S. Org. Lett. 2000, 2, 1887.
(b) Trost, B. M.; Ball, Z. T. J. Am. Chem. Soc. 2001, 123, 12726.
(c) Kawanami, Y.; Sonoda, Y.; Mori, T.; Yamamoto, K. Org. Lett. 2002, 4, 2825.
(d) Denmark, S. E.; Pan, W. Org. Lett. 2002, 4, 4163.
(e) Brunner, H. Angew. Chem., Int. Ed. 2004, 43, 2749.
(f) Aricó, C. S.; Cox, L. R. Org. Biomol. Chem. 2004, 2, 2558.
(g) Menozzi, C.; Dalko, P. I.; Cossy, J. J. Org. Chem. 2005, 70, 10717.
(h) Trost, B. M.; Ball, Z. T. J. Am. Chem. Soc. 2005, 127, 17644.
(i) Maifeld, S. V.; Tran, M. N.; Lee, D. Tetrahedron Lett. 2005, 46, 105.
(j) Berthon-Gelloz, G.; Schumers, J.-M.; De Bo, G.; Markó, I. E. J. Org. Chem. 2008, 73, 4190.
(k) Sore, H. F.; Blackwell, D. T.; MacDonald, S. J. F.; Spring, D. R. Org. Lett. 2010, 12, 2806.
(l) Ding, S.-T.; Song, L.-J.; Chung, L. W.; Zhang, X.-H.; Sun, J.-W.; Wu, Y.-D. J. Am. Chem. Soc. 2013, 135, 13835.
(m) Guo, J.; Lu, Z. Angew. Chem., Int. Ed. 2016, 55, 10835.
[4] (a) Pietraszuk, C.; Fischer, H.; Kujawa, M.; Marciniec, B. Tetrahedron Lett. 2001, 42, 1175.
(b) Denmark, S. E.; Yang, S.-M. Org. Lett. 2001, 3, 1749.
[5] (a) Takai, K.; Kataoka, Y.; Okazoe, T.; Utimoto, K. Tetrahedron Lett. 1987, 28, 1443.
(b) Murthi, K. K.; Salomon, R. G. Tetrahedron Lett. 1994, 35, 517.
(c) Hodgson, D. M.; Comina, P. J. Tetrahedron Lett. 1994, 35, 9469.
(d) Itami, K.; Nokami, T.; Yoshida, J.-I. Org. Lett. 2000, 2, 1299.
(e) McNulty, J.; Das, P. Chem. Commun. 2008, 1244.
(f) Mu, Q.-C.; Wang, X.-B.; Ye, F.; Sun, Y.-L.; Bai, X.-F.; Chen, J.; Xia, C.-G.; Xu, L.-W. Chem. Commun. 2018, 54, 12994.
[6] (a) Chen, C.; Hecht, M. B.; Kavara, A.; Brennessel, W. W.; Mercado, B. Q.; Weix, D. J.; Holland, P. L. J. Am. Chem. Soc. 2015, 137, 13244.
(b) Ibrahim, A. D.; Entsminger, S. W.; Zhu, L.-Y.; Fout, A. R. ACS Catal. 2016, 6, 3589.
(c) Jakobsson, K.; Chu, T.; Nikonov, G. I. ACS Catal. 2016, 6, 7350.
(d) Raya, B.; Jing, S.; Balasanthiran, V.; RajanBabu, T. V. ACS Catal. 2017, 7, 2275.
(e) Chen, Y.-J.; Ji, S.-F.; Sun, W.-M.; Chen, W.-X.; Dong, J.-C.; Wen, J.-F.; Zhang, J.; Li, Z.; Zheng, L.-R.; Chen, C.; Peng, Q.; Wang, D.-S.; Li, Y.-D. J. Am. Chem. Soc. 2018, 140, 7407.
(f) Liu, J.-X.; Chen, W.-F.; Li, J.-F.; Cui, C.-M. ACS Catal. 2018, 8, 2230.
[7] (a) Hori, Y.; Mrrsudo, T.; Watanabe, Y. Bull. Chem. Soc. Jpn. 1988, 61, 3011.
(b) Christ, M. L.; Sabo-Etienne, S.; Chaudret, B. Organometallics 1995, 14, 1082.
(c) Bokka, A.; Jeon, J. Org. Lett. 2016, 18, 5324.
[8] (a) Millan, A.; Towns, E.; Maitlis, P. M. J. Chem. Soc., Chem. Commun. 1981, 673.
(b) Millan, A.; Fernandez, M.-J.; Bentz, P.; Maitlis, P. M. J. Mol. Catal. 1984, 26, 89.
(c) Doyle, M. P.; Devora, G. A.; Nefedov, A. O.; High, K. G. Organometallics 1992, 11, 549.
(d) Takeuchi, R.; Yasue, H. Organometallics 1996, 15, 2098.
(e) Truscott, B. J.; Slawin, A. M. Z.; Nolan, S. P. Dalton Trans. 2013, 42, 270.
[9] LaPointe, A. M.; Rix, F. C.; Brookhart, M. J. Am. Chem. Soc. 1997, 119, 906.
[10] (a) Fernández, M. J.; Esteruelas, M. A.; Jlménez, M. S.; Oro, L. A. Organometallics 1986, 5, 1519.
(b) Oro, L. A.; Fernandez, M. J.; Esteruelas, M. A.; Jimenez, M. S. J. Mol. Catal. 1986, 37, 151.
(c) Tanke, R. S.; Crabtree, R. H. Organometallics 1991, 10, 415.
(d) Lu, B.; Falck, J. R. J. Org. Chem. 2010, 75, 1701.
(e) Cheng, C.; Simmons, E. M.; Hartwig, J. F. Angew. Chem., Int. Ed. 2013, 52, 8984.
[11] Sprengers, J. W.; de Greef, M.; Duin, M. A.; Elsevier, C. J. Eur. J. Inorg. Chem. 2003, 3811.
[12] Jiang, Y.; Blacque, O.; Fox, T.; Frech, C. M.; Berke, H. Chem.-Eur. J. 2009, 15, 2121.
[13] Nakamura, S.; Yonehara, M.; Uchiyama, M. Chem.-Eur. J. 2008, 14, 1068.
[14] (a) Sun, J.; Deng, L. ACS Catal. 2016, 6, 290.
(b) Du, X.-Y.; Huang, Z. ACS Catal. 2017, 7, 1227.
(c) Zhang, J.-H.; Hao, X.-Q.; Wang, Z.-L.; Ren, C.-J.; Niu, J.-L.; Song, M.-P. Chin. J. Org. Chem. 2017, 37, 1237 (in Chinese).(张家恒, 郝新奇, 王正龙, 任常久, 牛俊龙, 宋毛平, 有机化学, 2017, 37, 1237.)
(d) Gu, Z.-Y.; Ji, S.-J. Acta Chim. Sinica 2018, 76, 347 (in Chinese).(顾正洋, 纪顺俊, 化学学报, 2018, 76, 347.)
[15] Nesmeyanov, A. N.; Freidlina, R. K.; Chukovskaya, E. C.; Petrova, R. G.; Belyavsky, A. B. Tetrahedron 1962, 17, 61.
[16] (a) Kakiuchi, F.; Tanaka, Y.; Chatani, N.; Murai, S. J. Organomet. Chem. 1993, 456, 45.
(b) Naumov, R. N.; Itazaki, M.; Kamitani, M.; Nakazawa, H. J. Am. Chem. Soc. 2012, 134, 804.
(c) Marciniec, B.; Kownacka, A.; Kownacki, I.; Taylor, R. Appl. Catal. A:General 2014, 486, 230.
(d) Sunada, Y.; Noda, D.; Soejima, H.; Tsutsumi, H.; Nagashima, H. Organometallics 2015, 34, 2896.
(e) Du, X.-Y.; Zhang, Y.-L.; Peng, D.-J.; Huang, Z. Angew. Chem., Int. Ed. 2016, 55, 6671.
[17] (a) Marciniec, B.; Maciejewski, H.; Kownacki, I. J. Mol. Catal. A:Chemical 1998, 135, 223.
(b) Maciejewski, H.; Marciniec, B.; Kownacki, I. J. Organomet. Chem. 2000, 597, 175.
[18] Takeshita, K.; Seki, Y.; kawamoto, K.; Murai, S.; Sonoda, N. J. Org. Chem. 1987, 52, 4864.
[19] Atienza, C. C. H.; Diao, T.; Weller, K. J.; Nye, S. A.; Lewis, K. M.; Delis, J. G. P.; Boyer, J. L.; Roy, A. K.; Chirik, P. J. J. Am. Chem. Soc. 2014, 136, 12108.
[20] Gorczyński, A.; Zaranek, M.; Witomska, S.; Bocian, A.; Stefankiewicz, A. R.; Kubicki, M.; Patroniak, V.; Pawluc, P. Catal. Commun. 2016, 78, 71.
[21] (a) Wang, C.; Teo, W. J.; Ge, S.-Z. ACS Catal. 2017, 7, 855.
(b) Liu, Y.; Deng, L. J. Am. Chem. Soc. 2017, 139, 1798.
(c) Gao, Y.-F.; Wang, L.-J.; Deng, L. ACS Catal. 2018, 8, 9637.
(d) Basu, D.; Gilbert-Wilson, R.; Gray, D. L.; Rauchfuss, T. B. Organometallics 2018, 37, 2760.
(e) Sanagawa, A.; Nagashima, H. Organometallics 2018, 37, 2859.
[22] For selected reviews on earth-abundant transition metal-catalyzed asymmetric hydrofunctionalization of alkenes and alkynes, see:
(a) Chen, J.-H.; Lu, Z. Org. Chem. Front. 2018, 5, 260.
(b) Chen, J.-H.; Guo, J.; Lu, Z. Chin. J. Chem. 2018, 36, 1075.
[23] For hydroboration of alkenes and alkynes, see:(a) Chen, J.-H.; Xi, T.; Lu, Z. Org. Lett. 2014, 16, 6452.
(b) Chen, J.-H.; Xi, T.; Ren, X.; Cheng, B.; Guo, J.; Lu, Z. Org. Chem. Front. 2014, 1, 1306.
(c) Zhang, H.-Y.; Lu, Z. ACS Catal. 2016, 6, 6596.
(d) Xi, T.; Lu, Z. ACS Catal. 2017, 7, 1181.
(e) Chen, X.; Cheng, Z.-Y.; Lu, Z. Org. Lett. 2017, 19, 969.
(f) Chen, C.-H.; Shen, X.-Z.; Chen, J.-H.; Hong, X.; Lu, Z. Org. Lett. 2017, 19, 5422.
(g) Guo, J.; Cheng, B.; Shen, X.-Z.; Lu, Z. J. Am. Chem. Soc. 2017, 139, 15316.
(h) Chen, X.; Cheng, Z.-Y.; Guo, J.; Lu, Z. Nat. Commun. 2018, 9, 3939.
[24] For hydrosilylation of alkenes and alkynes, see:
(a) Chen, J.-H.; Cheng, B.; Cao, M.-Y.; Lu, Z. Angew. Chem., Int. Ed. 2015, 54, 4661.
(b) Guo, J.; Lu, Z. Angew. Chem., Int. Ed. 2016, 55, 10835.
(c) Xi, T.; Lu, Z. J. Org. Chem. 2016, 81, 8858.
(d) Guo, J.; Shen, X.-Z.; Lu, Z. Angew. Chem., Int. Ed. 2017, 56, 615.
(e) Cheng, B.; Lu, P.; Zhang, H.-Y.; Cheng, X.-P.; Lu, Z. J. Am. Chem. Soc. 2017, 139, 9439.
(f) Cheng, B.; Liu, W.-B.; Lu, Z. J. Am. Chem. Soc. 2018, 140, 5014.
(g) Cheng, Z.-Y.; Xing, S.-P.; Guo, J.; Cheng, B.; Hu, L.-F.; Zhang, X.-H.; Lu, Z. Chin. J. Chem. 2019, 37, 457.
(h) Guo, J.; Wang, H.-L.; Xing, S.-P. Hong, X.; Lu, Z. Chem 2019, 5, 881.
[25] For asymmetric hydrogenation of alkenes, see:Chen, J.-H.; Chen, C.-H.; Ji, C.-L.; Lu, Z. Org. Lett. 2016, 18, 1594.
[26] Zhang, L.; Zuo, Z.-Q.; Wan, X.-L.; Huang, Z. J. Am. Chem. Soc. 2014, 136, 15501.
[27] Komiyama, T.; Minami, Y.; Hiyama, T. ACS Catal. 2017, 7, 631.
[28] Guo, J.; Chen, J.-H.; Lu, Z. Chem. Commun. 2015, 51, 5725.
[29] JoongLee, S.; KyeuPark, M.; HeeHan, B. Silicon Chemistry 2002, 1, 41.
[30] Ramírez-Oliva, E.; Hernández, A.; Martínez-Rosales, J. M.; Aguilar-Elguezabal, A.; Herrera-Pérez, G.; Cervantes, J. ARKIVOC 2006, 126.
[31] Karabelas, K.; Hallberg, A. J. Org. Chem. 1986, 51, 5286.
/
| 〈 |
|
〉 |