

马来酰亚胺双键参与的官能化反应研究进展
收稿日期: 2019-02-14
修回日期: 2019-04-10
网络出版日期: 2019-04-19
基金资助
上海市自然科学基金(No.15ZR1401400)和东华大学国家级大学生创新性实验(2018)资助项目.
Recent Advances in Functionalization of Double Bond Based on Maleimides
Received date: 2019-02-14
Revised date: 2019-04-10
Online published: 2019-04-19
Supported by
Project supported by the Shanghai Municipal Natural Science Foundation (No. 15ZR1401400) and the National Undergraduate Training Program for Innovation and Entrepreneurship in Donghua University (2018).
杨振华 , 祝家楠 , 文彩月 , 葛迎香 , 赵圣印 . 马来酰亚胺双键参与的官能化反应研究进展[J]. 有机化学, 2019 , 39(9) : 2412 -2427 . DOI: 10.6023/cjoc201902012
Maleimide, a common motif in a variety of natural alkaloids, has been extensively investigated due to its noteworthy biological activities and optical properties. Additionally, it can be transformed into many important heterocyclic frameworks such as succinimides, pyrrolidines, and 2-pyrrolidones. Thus, a great deal of attention has been focused on the development of new synthetic routes to access polyfunctionalized maleimides. In this article, the recent research progress in functionalization of double bond is reviewed based on maleimides according to Michael addition, oxidative coupling and cycloaddition reaction.
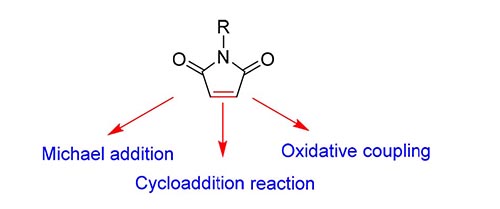
Key words: maleimide; Michael addition; oxidative coupling; cycloaddition reaction
[1] Lavrard, H.; Rodriguez, F.; Delfourne, E. Bioorg. Med. Chem. Lett. 2014, 22, 1961.
[2] Chien, S.-C.; Chen, M.-L.; Kuo, H.-T.; Tsai, Y.-C.; Lin, B.-F.; Kuo, Y.-H. J. Agric. Food Chem. 2008, 56, 7017.
[3] (a) Ho, S.-Y.; Alam, J.; Jeyaraj, D.-A.; Wang, W.; Lin, G.-R.; Ang, S.-H.; Tan, E.-S.-W.; Lee, M.-A.; Ke, Z.; Madan, B.; Virshup, D.-M.; Ding, L.-J.; Manoharan, V.; Chew, Y.-S.; Low, C.-B.; Pendharkar, V.; Sangthongpitag, K.; Hill, J.; Keller, T.-H.; Poulsen, A. J. Med. Chem. 2017, 60, 6678.
(b) Alam, J.; Poulsen, A.; Ho, S.-Y.; Wang, W.-L.; Duraiswamy, A. WO 2015094118, 2008[Chem. Abstr. 2015, 163, 132777].
[4] Kayser, S.; Levis, M.-J.; Schlenk, R.-F. Expert Rev. Clin. Pharmacol. 2017, 10, 1177.
(b) Levis, M. Blood 2017, 129, 3403.
[5] Shimokawa, J.; Chiyoda, K.; Umihara, H.; Fukuyama, T. Chem. Pharm. Bull. 2016, 64, 1239.
(b) Cai, S.-L.; Song, R.; Dong, H.-Q.; Lin, G.-Q.; Sun, X.-W. Org. Lett. 2016, 18, 1996.
[6] (a) Daly, M.-J.; Jones, G.-W.; Nicholls, P.-J.; Smith, H.-J.; Rowlands, M.-G.; Bunnett, M.-A. J. Med. Chem. 1986, 29, 520.
(b) Sharma, D.-K.; Rajput, V.-S.; Singh, S.; Sharma, R.; Khan, I. A.; Mukherjee, D. ChemistrySelect 2016, 1, 1954.
[7] (a) Driller, K.-M.; Klein, H.; Jackstell, R.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 6041.
(b) Mathur, P.; Joshi, R.-K.; Rai, D.-K.; Jha, B.; Mobin, S.-M. Dalton. Trans. 2012, 41, 5045.
[8] Henon, H.; Messaoudi, S.; Hugon, B.; Anizon, F.; Pfeiffer, B.; Prudhomme, M. Tetrahedron 2005, 61, 5599.
[9] An, Y.-L.; Shao, Z.-Y.; Cheng, J.; Zhao, S.-Y. Synthesis 2013, 45, 2719.
[10] Lanke, V.; Bettadapur, K.-R.; Prabhu, K.-R. Org. Lett. 2015, 17, 4662.
[11] Muniraj, N.; Prabhu, K.-R. ACS Omega 2017, 2, 4470.
[12] Zhang, Z.; Han, S.; Tang, M.; Ackermann, L.; Li, J. Org. Lett. 2017, 19, 3315.
[13] Liu, S.-L.; Li, Y.; Guo, J.-R.; Yang, G.-C.; Li, X.-H.; Gong, J.-F.; Song, M.-P. Org. Lett. 2017, 19, 4042.
[14] Sherikar, M.-S.; Kapanaiah, R.; Lanke, V.; Prabhu, K.-R. Chem. Commun. 2018, 54, 11200.
[15] Pan, C.; Wang, Y.; Wu, C.; Yu, J.-T. Org. Biomol. Chem. 2018, 16, 693.
[16] Koltunov, K.-Y.; Prakash, G.-K.-S.; Rasul, G.; Olah, G.-A. Eur. J. Org. Chem. 2006, 4861.
[17] Yang, Z.-H.; Chen, Z.-H.; An, Y.-L.; Zhao, S.-Y. RSC Adv. 2016, 6, 23438.
[18] Bettadapur, K.-R.; Lanke, V.; Prabhu, K.-R. Org. Lett. 2015, 17, 4658.
[19] Mandal, R.; Emayavaramban, B.; Sundararaju, B. Org. Lett. 2018, 20, 2835.
[20] Li, F.; Zhou, Y.; Yang, H.; Liu, D.; Sun, B.; Zhang, F.-L. Org. Lett. 2018, 1, 146.
[21] Yu, J. T.; Chen, R.; Jia, H.; Pan, C. J. Org. Chem. 2018, 83, 12086.
[22] Han, S.-H.; Kim, S.; De, U.; Mishra, N.-K.; Park, J.; Sharma, S.; Kwak, J.-H.; Han, S.; Kim, H.-S.; Kim, I.-S. J. Org. Chem. 2016, 81, 12416.
[23] He, Q.; Yamaguchi, T.; Chatani, N. Org. Lett. 2017, 19, 4544.
[24] Chen, X.; Ren, J.; Xie, H.; Sun, W.; Sun, M.; Wu, B. Org. Chem. Front. 2018, 5, 184.
[25] Bettadapur, K.-R.; Lanke, V.; Prabhu, K.-R. Chem. Commun. 2017, 53, 6251.
[26] Mandal, A.; Sahoo, H.; Dana, S.; Baidya, M. Org. Lett. 2017, 19, 4138.
[27] Muniraj, N.; Prabhu, K.-R. J. Org. Chem. 2017, 82, 6913.
[28] Qrareya, H.; Ravelli, D.; Fagnoni, M.; Albinia, A. Adv. Synth. Catal. 2013, 355, 2891.
[29] Capaldo, L.; Buzzetti, L.; Merli, D.; Fagnoni, M.; Ravelli, D. J. Org. Chem. 2016, 81, 7102.
[30] Han, S.; Park, J.; Kim, S.; Lee, S.-H.; Sharma, S.; Mishra, N.-K.; Jung, Y.-H.; Kim, I.-S. Org. Lett. 2016, 18, 4666.
[31] (a) Cunha, S.; Rodovalho, W.; Azevedo, N. R.; Mendonca, M.-D.-O.; Lariucci, C.; Vencato, I. J. Brazil. Chem. Soc. 2002, 13, 629.
(b) Gomez-Torres, E.; Alonso, D.-A.; Gomez-Bengoa, E.; Najera, C. Eur. J. Org. Chem. 2013, 2013, 1434.
(c) Noeth, J.; Frankowski, K.-J.; Neuenswander, B.; Aube, J.; Reiser, O. J. Comb. Chem. 2008, 10, 456.
[32] Zhao, G.-L.; Xu, Y.-M.; Sunden, H.; Eriksson, L.; Sayah, M.; Cordova, A. Chem. Commun. 2007, 7, 734.
[33] Yu, F.; Jin, Z.; Huang, H.; Ye. T.; Liang, X.; Ye, J.-X. Org. Bio-mol. Chem. 2010, 8, 4767.
[34] Yu, F.; Sun, X.; Jin, Z.; Wen, S.; Liang, X.; Ye, J.-X. Chem. Commun. 2010, 46, 4589.
[35] Muramulla, S.; Ma, J.-A.; Zhao, J.-C.-G. Adv. Synth. Catal. 2013, 355, 1260.
[36] Vizcaino-Milla, P.; Sansano, J.-M.; Najera, C.; Fiser, B.; Gomez-Bengoa, E. Synthesis 2015, 47, 2199.
[37] Nakashima, K.; Kawada, M.; Hirashima, S.; Kato, M.; Koseki, Y.; Miura, T. Synlett 2015, 26, 1248.
[38] Wang, J.-J.; Dong, X.-J.; Wei, W.-T.; Yan, M. Tetrahedron:Asymmetry 2011, 22, 690.
[39] Bai, J.-F.; Wang, L.-L.; Peng, L.; Guo, Y.-L.; Jia, L.-N.; Tian, F.; He, G.-Y.; Xu, X.-Y.; Wang, L.-X. J. Org. Chem. 2012, 77, 2947.
[40] Shirakawa, S.; Terao, S. J.; He, R.; Maruoka, K. Chem. Commun. 2011, 47, 10557.
[41] Gomez-Torres, E.; Alonso, D. A.; Gomez-Bengoa, E.; Najera, C. Org. Lett. 2011, 13, 6106.
[42] Li, X.; Hu, S.; Xi, Z.; Zhang, L.; Luo, S.; Cheng, J.-P. J. Org. Chem. 2010, 75, 8697.
[43] Liao, Y.-H.; Liu, X.-L.; Wu, Z.-J.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Org. Lett. 2010, 12, 2896.
[44] Feng, J.; Zhang, Y.; Lin, L.; Yao, Q.; Liu, X.; Feng, X. Chem. Commun. 2015, 51, 10554.
[45] Yarlagadda, S.; Reddy, C.-R.; Ramesh, B.; Kumar, G.-R.; Sridhar, B.; Reddy, B.-V.-S. Eur. J. Org. Chem. 2018, 1364.
[46] Li, J.; Qiu, S.; Ye, X.; Zhu, B.; Liu, H.; Jiang, Z. J. Org. Chem. 2016, 81, 11916.
[47] Iyer, P.-S.; O'Malley, M.-M.; Lucas, M.-C. Tetrahedron Lett. 2007, 48, 4413.
[48] Shintani, R.; Duan, W.-L.; Nagano, T.; Okada, A.; Hayashi, T. Angew. Chem., Int. Ed. 2005, 44, 4611.
[49] Berhal, F.; Wu, Z.; Genet, J.; Ayad, T.; Ratovelomanana-Vidal, V. J. Org. Chem. 2011, 76, 6320.
[50] Korenaga, T.; Ko, A.; Shimamda, K. J. Org. Chem. 2013, 78, 9975.
[51] Gopula, B.; Yang, S.-H.; Kuo, T.-S.; Hsieh, J.-C.; Wu, P.-Y.; Henschke, J.-P.; Wu, H.-L. Chem. Eur. J. 2015, 21, 11050.
[52] Kumar, V.; Mitra, R.; Bhattarai, S.; Nair, V.-A. Synth. Commun. 2011, 41, 392.
[53] Raycroft, M.-A.-R.; Racine, K.-E.; Rowley, C.-N.; Keillor, J.-W. J. Org. Chem. 2018, 83, 11674.
[54] Han, F.; Yang, L.; Li, Z.; Xia, C.-G. Org. Biomol. Chem. 2012, 10, 346.
[55] An, Y.-L.; Deng, Y.-X.; Zhang, W.; Zhao, S.-Y. Synthesis 2015, 47, 1581.
[56] Velchinskaya, E.; Petsushak, B.; Rogal, A. Chem. Heterocycl. Compd. 2007, 43, 695.
[57] Uno, B.-E.; Deibler, K.-K.; Villa, C.; Raghuraman, A.; Scheidt, K.-A. Adv. Synth. Catal. 2018, 360, 1719.
[58] Uno, B.-E.; Dicken, R.-D.; Redfern, L.-R.; Stern, C.-M.; Krzywicki, G.-G.; Scheidt, K.-A. Chem. Sci. 2018, 9, 1634.
[59] Jiang, Z.; Zhang, Y.; Ye, W.; Tan, C.-H. Tetrahedron Lett. 2007, 48, 51.
[60] Balint, E.; Takacs, J.; Drahos, L.; Keglevich, G. Heteroat. Chem. 2012, 23, 235.
[61] Molleti, N.; Bjornberg, C.; Kong, J.-Y. Org. Biomol. Chem. 2016, 14, 10695.
[62] (a) Bourderioux, A.; Routier, S.; Beneteau, V.; Merour, J.-Y. Tetrahedron 2007, 63, 9465.
(b) Bouissane, L.; Sestelo, J.-P.; Sarandeses, L. A. Org. Lett. 2009, 11, 1285.
(c) Awuah, E.; Capretta, A. J. Org. Chem. 2011, 76, 3122.
(d) Souffrin, A.; Croix, C.; Viaud-Massuard, M.-C. Eur. J. Org. Chem. 2012, 13, 2499
[63] Roshchin, A.-I.; Polunin, E.-V. Mendeleev Commun. 2008, 18, 332.
[64] Lim, L.-H.; Zhou, J. Org. Chem. Front. 2015, 2, 775.
[65] Jafarpour, F.; Shamsianpour, M.; Issazadeh, S.; Dorrani, M.; Hazrati, H. Tetrahedron 2017, 73, 1668.
[66] Jafarpour, F.; Shamsianpour, M. RSC Adv. 2016, 6, 103567.
[67] Yang, Z.-H.; An, Y.-L.; Chen, Y.; Shao, Z.-Y.; Zhao, S.-Y. Adv. Synth. Catal. 2016, 358, 3869.
[68] Dana, S.; Mandal, A.; Sahoo, H.; Baidya, M. Org. Lett. 2017, 19, 1902.
[69] An, Y.-L.; Zhang, H.-H.; Yang, Z.-H.; Lin, L.; Zhao, S.-Y. Eur. J. Org. Chem. 2016, 2016, 5405.
[70] Kong, D.-H.; An, Y.-L.; Shao, Z.-Y.; Zhao, S.-Y. J. Chem. Res. 2018, 42, 476.
[71] (a) Yang, Z.-H.; Tan, H.-R.; An, Y.-L.; Zhao, Y.-W.; Lin, H.-P.; Zhao, S.-Y. Adv. Synth. Catal. 2018, 360, 173.
(b) Yang, Z.-H.; Zhu, J.-N.; Jin, Z.-H.; Zheng, J.; Zhao, S.-Y. Synthesis 2018, 50, 4627.
[72] Yang, Z.-H.; Tan, H.-R.; Zhu, J.-N.; Zheng, J.; Zhao, S.-Y. Adv. Synth. Catal. 2018, 360, 1523.
[73] (a) Maruoka, H.; Okabe, F.; Koutake, Y.; Fujioka, T.; Yamagata, K. Heterocycles 2009, 77, 617.
(b) Bai, J.-F.; Guo, Y.-L.; Peng, L.; Jia, L.-N.; Xu, X.-Y.; Wang, L.-X. Tetrahedron 2013, 69, 1229.
(b) Petrelli, A.; Samain, E.; Pradeau, S.; Halila, S.; Fort, S. ChemBioChem 2017, 18, 206.
[74] Baker, J.-R.; Tedaldi, L.-M.; Aliev, A.-E. Chem. Commun. 2012, 48, 4725.
[75] Lin, C.; Zhen, L.; Cheng, Y.; Du, H.-J.; Zhao, H.; Wen, X.; Kong, L.-Y.; Xu, Q.-L.; Sun, H. Org. Lett. 2015, 17, 2684.
[76] Ding, G.; Wu, X.; Jiang, L.; Zhang, Z.; Xie, X. Org. Lett. 2017, 19, 6048.
[77] Qiu, S.; Lee, R.; Zhu, B.; Coote, M.-L.; Zhao, X.; Jiang, Z. J. Org. Chem. 2016, 81, 8061.
[78] Nishikawa, Y.; Nakano, S.; Tahira, Y.; Terazawa, K.; Yamazaki, K.; Kitamura, C.; Hara, O. Org. Lett. 2016, 18, 2004.
[79] Kumar, G.-V.; Govindaraju, M.; Renuka, N.; Khatoon, B.-B.-A.; Mylarappa, B.-N.; Kumar, K.-A. Rasayan J. Chem. 2012, 5, 338.
[80] Liu, G.-N.; Luo, R.-H.; Zhou, Y.; Zhang, X.-J.; Li, J.; Yang, L.-M.; Zheng, Y.-T.; Liu, H. Molecules 2016, 21, 1198/1.
[81] Zhang, X.-N.; Chen, G.-Q.; Tang, X.-Y.; Wei, Y.; Shi, M. Angew. Chem., Int. Ed. 2014, 53, 10768.
[82] Sharma, S.; Han, S. H.; Oh, Y.; Mishra, N.-K.; Lee, S.-H.; Oh, J.-S.; Kim, I.-S. Org. Lett. 2016, 18, 2568.
[83] Morita, T.; Akita, M.; Satoh, T.; Kakiuchi, F.; Miura, M. Org. Lett. 2016, 18, 4598.
[84] Yu, W.; Zhang, W.; Liu, Y.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2017, 4, 77.
[85] Zhu, C.; Falck, J.-R. Chem. Commun. 2012, 48, 1674.
[86] Miura, W.; Hirano, K.; Miura, M. Org. Lett. 2015, 17, 4034.
/
| 〈 |
|
〉 |