

磷脂酰基醇3-激酶抑制剂甲磺酸普喹替尼的合成
收稿日期: 2019-02-20
修回日期: 2019-03-25
网络出版日期: 2019-04-19
基金资助
重大新药创制(No.2008ZX09101-048)资助项目.
Development of Synthesis of Phosphatidylinositol 3-Kinases Inhibitor Puquitinib Mesylate
Received date: 2019-02-20
Revised date: 2019-03-25
Online published: 2019-04-19
Supported by
Project supported by the Significant New Drugs Development (No. 2008ZX09101-048).
沈大冬 , 朱金林 , 吴国锋 , 盛力 , 高浩凌 , 王普 . 磷脂酰基醇3-激酶抑制剂甲磺酸普喹替尼的合成[J]. 有机化学, 2019 , 39(9) : 2676 -2680 . DOI: 10.6023/cjoc201902020
Puquitinib mesylate is a novel phosphatidylinositol 3-kinases (PI3K) inhibitor, which has been shown to be effective in the treatment of cancer. A convenient protocol for the synthesis of the compound at kilogram scale is described, using 2,6-dichloropurine as starting material through amino-protection, SN2 reaction with high regioselectivity, Buchwald-Hartwig coupling reaction, amino-deprotection and salt-forming reaction. The process is easy to operate and provides an effective way at kilogram scale produce with 48% yield in total.
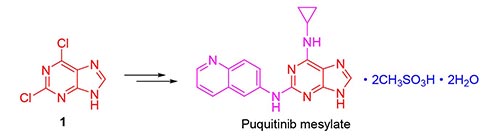
Key words: PI3K inhibitor; puquitinib; synthesis
[1] (a) Thomas, M.; Owen, C. Curr. Opin. Pharmacol. 2008, 8, 267.
(b) Marone, R.; Cmiljanovic, V.; Giese, B.; Wymann, M. P. Biochim. Biophys. Acta, Proteins Proteomics 2008, 1784, 159.
(c) Shuttleworth, S.; Silva, F.; Tomassi, C.; Cecil, A.; Hill, T.; Rogers, H.; Townsend, P. Prog. Med. Chem. 2009, 48, 81.
[2] (a) Vlahos, C. J.; Matter, W. F.; Hui, K. Y.; Brown, R. F. J. Biol. Chem. 1994, 269, 5241.
(b) Walker, E. H.; Pacold, M. E.; Perisic, O.; Stephens, L.; Hawkins, P.; Wymann, M. Williams, R. L. Mol. Cell. 2000, 6, 909.
[3] (a) Ameriks, M. K.; Venable, J. D. Curr. Top. Med. Chem. 2009, 9, 738.
(b) Welker, M. E.; Kulik, G. Bioorg. Med. Chem. 2013, 21, 4063.
(c) Wei, M.-M.; Wang, X.; Song, Z.-L.; Jiao, M.-K.; Ding, J.; Meng, L.-H.; Zhang, A. Med. Res. Rev. 2015, 35, 720.
(d) Musiol R. Expert Opin. Drug Discovery 2017, 12, 583.
[4] Xie, C.-Y.; Xu, Y.; Lou, L.-G. Cancer Res. 2011, 71, 4245.
[5] Wang, K.-F.; Yang, H.; Jiang, W.-Q.; Li, S.; Cai, Y.-C. Int. J. Mol. Med. 2015, 36, 1556.
[6] Xie, C. Y.; He, Y.; Zhen, M. Y.; Wang, Y. L.; Xu, Y. P.; Lou, L. G. Cancer Sci. 2017, 108, 1476.
[7] Yang, H.; Wang, Y.; Zhan, J.; Xia, Y.; Sun, P.; Bi, X.-W.; Liu, P.-P.; Li, Z.-M.; Li, S.; Zou, B.-Y.; Jiang, W.-Q. Oncotarget 2015, 6, 44049.
[8] Ding, Y.; Zhan, Q.; Wu, Y.-H.; Zou, B.-Y.; Li, Z.-M.; Li, S.; Jiang, W.-Q. J. Sun Yat-Sen Univ. Med. Sci. 2015, 36, 414(in Chinese). (丁娅, 詹靖, 吴跃翰, 吴跃翰, 邹本燕, 李志铭, 李苏, 姜文奇. 中山大学学报(医学科学版), 2015, 36, 414.)
[9] Wu, Z.-G. WO 2006133611, 2006.
[10] (a) Murray, J. M.; Sweeney, Z. K.; Chan, B. K.; Balazs, M.; Bradley, E.; Castanedo, G.; Chabot, C.; Chantry, D.; Flagella, M.; Goldstein, D. M.; Kondru, R.; Lesnick, J.; Li, J.; Lucas, M. C.; Nonomiya, J.; Pang, J.; Price, S.; Salphati, L.; Safina, B.; Savy, P. P. A.; Seward, E. M.; Ultsch, M.; Sutherlin, D. P. J. Med. Chem. 2012, 55, 7686.
(b) Kenny, J. R.; Krintel, S. L.; Li, J.; Lesnick, J.; Lucas, M. C.; Lewis, C.; Mukadam, S.; Murray, J.; Nadin, A. J.; Nonomiya, J.; Padilla, F.; Palmer, W. S.; Pang, J.; Pegg, N.; Price, S.; Reif, K.; Salphati, L.; Savy, P. A.; Seward, E. M.; Shuttleworth, S.; Sohal, S.; Sweeney, Z. K.; Tay, S.; Tivitmahaisoon, P.; Waszkowycz, B.; Wei, B.; Yue, Q.; Zhang, C.; Sutherlin, D. P. J. Med. Chem. 2012, 55, 5887.
[11] Blanchard, S.; Soh, C. K.; Lee, C. P.; Poulsen, A.; Bonday, Z.; Goh, K. L.; Goh, K. C.; Goh, M. K.; Pasha, M. K.; Wang, H. S.; Williams, M.; Wood, J. M.; Ethirajulu, K.; Dymock, B. W. Bioorg. Med. Chem. Lett. 2012, 22, 2880.
[12] Fiorini, M. T.; Abell, C. Tetrahedron Lett. 1998, 39, 1827
[13] Beletskaya, I. P.; Cheprakov, A. V. Organometallics 2012, 31, 7753
[14] Burger, M. T.; Knapp, M.; Wagman, A.; Ni, Z. J.; Hendrickson, T.; Atallah, G.; Zhang, Y.; Frazier, K.; Verhagen, J.; Pfister, K.; Ng, S.; Smith, A.; Bartulis, S.; Merrit, H.; Weismann, M.; Xin, X.; Haznedar, J.; Voliva, C. F.; Iwanowicz, E.; Pecchi, S. ACS Med. Chem. Lett. 2011, 2, 34.
[15] Giordanetto, F.; Wållberg, A.; Cassel, J.; Ghosal, S.; Kossenjans, M.; Yuan, Z. Q.; Wang, X.; Liang, L. Bioorg. Med. Chem. Lett. 2012, 22, 6665.
/
| 〈 |
|
〉 |