

含树枝状和3D三苯胺衍生物有机染料的合成与光伏性能研究
收稿日期: 2019-02-21
修回日期: 2019-04-14
网络出版日期: 2019-04-19
基金资助
国家自然科学基金(Nos.21875204,51173154)资助项目.
Synthesis and Photovoltaic Properties of Organic Dyes Containing Dendritic and 3D Triphenylamine Derivatives
Received date: 2019-02-21
Revised date: 2019-04-14
Online published: 2019-04-19
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21875204, 51173154).
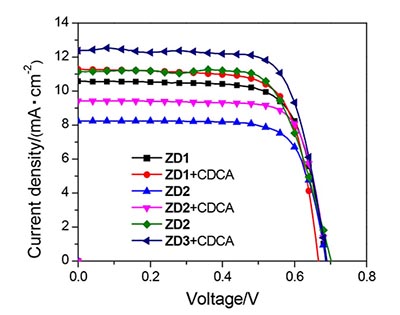
设计合成了三种以树枝状和3D三苯胺衍生物为给体单元,苯甲酸为受体单元,苯并噻二唑(BT)或双氟苯并噻二唑(DFBT)作为第二受体单元的有机染料,系统研究了不同给体和第二受体单元对染料敏化剂的光物理性能、电化学性能和光伏性能的影响.以树枝状三苯胺衍生物为给体单元的有机染料具有较高的摩尔吸收系数,以3D三苯胺衍生物(IDTTPA)为给体单元的有机染料具有较宽的吸收光谱.基于三种有机染料的染料敏化太阳能电池分别获得了5.27%,4.22%和5.50%的能量转换效率.采用1 mmol·L-1鹅去氧胆酸(CDCA)共吸附剂对电池进行优化后,三种染料的能量转换效率分别提升到5.46%,4.98%和6.26%.
周鑫云 , 谢凌超 , 吴凯乐 , 谭松庭 . 含树枝状和3D三苯胺衍生物有机染料的合成与光伏性能研究[J]. 有机化学, 2019 , 39(9) : 2589 -2598 . DOI: 10.6023/cjoc201902023
Three organic dyes with dendritic and 3D triphenylamine derivatives as the donor unit, benzoic acid as the acceptor unit and benzothiadiazole (BT) or difluorobenzothiadiazole (DFBT) as the second acceptor were designed and synthesized. The influences of different donors and second acceptors on the photophysical, electrochemical and photovoltaic properties of dye-sensitizers were systematically investigated. The organic dye with dendritic triphenylamine derivative as a donor unit possesses a higher molar absorption coefficient, and the organic dye with 3D triphenylamine derivative (IDTTPA) as a donor unit has a broader absorption spectrum. The dye-sensitized solar cells based on three organic dyes achieved power conversion efficiencies of 5.27%, 4.22% and 5.50%, respectively. After optimizing the battery with 1 mmol·L-1 co-adsorbent chenodeoxycholic acid (CDCA), the power conversion efficiencies of the organic dyes were increased to 5.46%, 4.98% and 6.26%, respectively.

Key words: triphenylamine; benzothiadiazole; organic dye; photovoltaic property
[1] O'regan, B.; Grätzel, M. Nature 1991, 353, 737.
[2] Hagfeldt, A.; Boschloo, G.; Sun, L. C.; Kloo, L.; Pettersson, H. Chem. Rev. 2010, 110, 6595.
[3] Clifford, J. N.; Martínez-Ferrero, E.; Viterisi, A.; Palomares, E. Chem. Soc. Rev. 2011, 40, 1635.
[4] Wu, Y.-Z.; Zhu, W. H. Chem. Soc. Rev. 2013, 42, 2039.
[5] Kou, D.-X.; Liu, W.-Q.; Hu, L.-H.; Chen, S.-H.; Huang, Y.; Dai, S.-Y. Acta Chim. Sinica 2013, 71, 1149(in Chinese). (寇东星, 刘伟庆, 胡林华, 陈双宏, 黄阳, 戴松元, 化学学报, 2013, 71, 1149.)
[6] Feng, X.-M.; Huang, X.-W.; Tan, Z.; Zhao, B.; Tan, S.-T. Acta Chim. Sinica 2011, 69, 653(in Chinese). (冯小明, 黄先威, 谭卓, 赵斌, 谭松庭, 化学学报, 2011, 69, 653.)
[7] Huang, X.-W.; Deng, J.-Y.; Xu, L.; Shen, P.; Zhao, B.; Tan, S.-T. Acta Chim. Sinica 2012, 70, 1604(in Chinese). (黄先威, 邓继勇, 许律, 沈平, 赵斌, 谭松庭, 化学学报, 2012, 70, 1604.)
[8] Li, J.; Kong, F.-T.; Zhang, C.-N.; Liu, W.-Q.; Dai, S.-Y. Acta Chim. Sinica 2010, 68, 1357(in Chinese). (李洁, 孔凡太, 张昌能, 刘伟庆, 戴松元, 化学学报, 2010, 68, 1357.)
[9] Tang, X.; Wang, Y.-X. Acta Chim. Sinica 2013, 71, 193(in Chinese). (唐笑, 汪禹汛, 化学学报, 2013, 71, 193.)
[10] Wang, J.-W.; Li, Y.; Xu, Y.-L.; Li, Y.; Shen, K.-H. Acta Chim. Sinica 2012, 70, 1278(in Chinese). (王纪伟, 李莹, 徐艳玲, 李杨, 申凯华, 化学学报, 2012, 70, 1278.)
[11] He, J.-J.; Chen, S.-X.; Wang, T.-T.; Zeng, H.-P. Chin. J. Org. Chem. 2012, 32, 472(in Chinese). (何俊杰, 陈舒欣, 王婷婷, 曾和平, 有机化学, 2012, 32, 472.)
[12] Cao, Y.-M.; Bai, Y.; Yu, Q.-J.; Cheng, Y.-M.; Liu, S.; Shi, D.; Gao, F.-F.; Wang, P. J. Phys. Chem. C 2009, 113, 6290.
[13] Yella, A.; Mai, C. L.; Zakeeruddin, S. M.; Chang, S. N.; Hsieh, C. H.; Yeh, C. Y.; Grätzel, M. Angew. Chem., Int. Ed. 2014, 53, 2973.
[14] Gupta, K.; Singh, S. P.; Islam, A.; Han, L.; Chandrasekharam, M. Electrochim. Acta 2015, 174, 581.
[15] Mishra, A.; Fischer, M. K.; Bäuerle, P. Angew. Chem., Int. Ed. 2009, 48, 2474.
[16] Han, L.; Zhou, X.; Ye, Q.; Li, Y.-J.; Gao, J.-R. Chin. J. Org. Chem. 2013, 33, 1000(in Chinese). (韩亮, 周雪, 叶青, 李郁锦, 高建荣, 有机化学, 2013, 33, 1000.)
[17] Wang, Z.-S.; Cui, Y.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Hara, K. J. Phys. Chem. C 2008, 112, 17011.
[18] Su, J.-Y.; Chen, Y.; Wu, Y.-G.; Ghimire, R. P.; Xu, Y.-J.; Liu, X.-J.; Wang, Z.-H.; Liang, M. Electrochim. Acta 2017, 254, 191.
[19] Han, L.; Wu, L.; Tong, Y.-Z.; Zu, X.-Y.; Jiang, S.-L. Chin. J. Org. Chem. 2017, 37, 2940(in Chinese). (韩亮, 吴靓, 童永正, 祖晓燕, 蒋绍亮, 有机化学, 2017, 37, 2940.)
[20] Chen, S.-G.; Jia, H.-L.; Ju, X.-H.; Zheng, H.-G. Dyes Pigm. 2017, 146, 127.
[21] Zhu, B.-Y.; Wu, L.; Ye, Q.; Gao, J.-R.; Han, L. Tetrahedron 2017, 73, 6307.
[22] Liang, M.; Xu, Y.-J.; Wang, X.-D.; Liu, X.-J.; Sun, Z.; Xue, S. Acta Chim. Sinica 2011, 69, 2092(in Chinese). (梁茂, 徐英军, 王旭达, 刘秀杰, 孙喆, 薛松, 化学学报, 2011, 69, 2092.)
[23] Cao, Z.-C.; He, Z.; Deng, L.-J.; Tan, S.-T. Chin. J. Org. Chem. 2014, 34, 340(in Chinese). (曹镇财, 何舟, 邓利军, 谭松庭, 有机化学, 2014, 34, 340.)
[24] Li, Q.-Q.; Shi, J.; Li, H.-Y.; Li, S.; Zhong, C.; Guo, F.-L.; Peng, M.; Hua, J.-L.; Qin, J.-G.; Li, Z. J. Mater. Chem. 2012, 22, 6689.
[25] Ito, S.; Miura, H.; Uchida, S.; Takata, M.; Sumioka, K.; Liska, P.; Comte, P.; Péchy, P.; Grätzel, M. Chem. Commun. 2008, 5194.
[26] Wu, Y.-Z.; Zhang, X.; Li, W.-Q.; Wang, Z.-S.; Tian, H.; Zhu, W.-H. Adv. Energy Mater. 2012, 2, 149.
[27] Li, Q.-Q.; Lu, L.-L.; Zhong, C.; Shi, J.; Huang, Q.; Jin, X.-B.; Peng, T.-Y.; Qin, J.-G.; Li, Z. J. Phys. Chem. B 2009, 113, 14588.
[28] Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Chem. Commun. 2015, 51, 15894.
[29] Liu, Z.-X.; Duan, K.-K.; Guo, H.; Deng, Y.-H.; Huang, H.-L.; Yi, X.-Y.; Chen, H.-J.; Tan, S.-T. Dyes Pigm. 2017, 140, 312.
[30] Zhu, H.-B.; Li, W.-Q.; Wu, Y.-Z.; Liu, B.; Zhu, S.-Q.; Li, X.; Agren, H.; Zhu, W.-H. ACS Sustainable Chem. Eng. 2014, 2, 1026.
[31] Han, L.; Zu, X.-Y.; Cui, Y.-H.; Wu, H.-B.; Ye, Q.; Gao, J.-R. Org. Electron. 2014, 15, 1536.
[32] Pan, B.; Zhu, Y.-Z.; Qiu, C.-J.; Wang, B.; Zheng, J.-O. Acta Chim. Sinica 2018, 76, 215(in Chinese). (潘彬, 朱义州, 邱昌娟, 王冰, 郑健禺, 化学学报, 2018, 76, 215.)
[33] Chen, H.-J.; Huang, H.; Huang, X.-W.; Clifford, J. N.; Forneli, A.; Palomares, E.; Zheng, X.-Y.; Zheng, L.-P.; Wang, X.-Y.; Shen, P.; Zhao, B.; Tan, S.-T. J. Phys. Chem. C 2010, 114, 3280.
[34] Huang, H.-L.; Chen, H.-J.; Long, J.; Wang, G.; Tan, S.-T. J. Power Sources 2016, 326, 438.
[35] Forster, F.; Rendón López, V.-M.; Oestreich, M. J. Am. Chem. Soc. 2018, 140, 1259.
[36] Song, X.-R.; Zhang, W.-W.; Li, X.; Jiang, H.-Y.; Shen, C.; Zhu, W.-H. J. Mater. Chem. C 2016, 4, 9203.
[37] Liu, Y.-H.; Cao, Y.-M.; Zhang, W.-W.; Stojanovic, M.; Dar, M I.; Péchy, P.; Saygili, Y.; Hagfeldt, A.; Zakeeruddin, S. M.; Grätzel, M. Angew. Chem., Int. Ed. 2018, 130, 14321.
[38] Chen, R.-K.; Yang, X.-C.; Tian, H.-N.; Sun, L.-C. J. Photochem. Photobiol. A 2007, 189, 295.
[39] Shen, P.; Liu, Y.-J.; Huang, X.-W.; Zhao, B.; Xiang, N.; Fei, J.-J.; Liu, L.-M.; Wang, X.-Y.; Huang, H.; Tan, S.-T. Dyes Pigm. 2009, 83, 187.
[40] Liu, X.-S.; Cao, Z.-C.; Huang, H.-L.; Liu, X.-X.; Tan, Y.-Z.; Chen, H.-J.; Pei, Y.; Tan, S.-T. J. Power Sources 2014, 248, 400.
[41] Hara, K.; Sato, T.; Katoh, R.; Furube, A.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. J. Phys. Chem. B 2003, 107, 597.
/
| 〈 |
|
〉 |