

3-去羟基Phomonol的合成
收稿日期: 2019-03-08
修回日期: 2019-04-18
网络出版日期: 2019-04-26
基金资助
国家自然科学青年基金(No.21302129)和浙江省自然科学基金(No.LQ13B020002)资助项目.
Synthesis of 3-Dehydroxyphomonol
Received date: 2019-03-08
Revised date: 2019-04-18
Online published: 2019-04-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 21302129) and the Natural Science Foundation of Zhejiang Province (No. LQ13B020002).
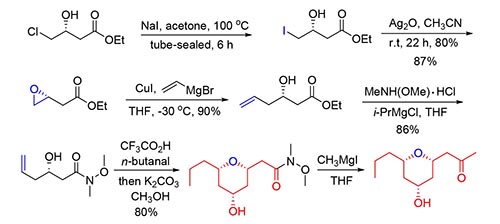
以商业购买的(R)-3-羟基-4-氯丁酸乙酯或者(S)-1,2-环氧戊烷为原料,经过6步反应,完成了天然产物类似物3-去羟基phomonol的合成,关键步骤包括格氏反应,Prins环化反应和钯催化分子内烷氧羰基化反应等,无保护基合成策略的应用缩短了合成步骤,提高了合成的效率.
关键词: 合成; 3-去羟基phomonol; Prins环化反应; 钯催化分子内烷氧羰基化反应
叶青青 , 张梦帆 , 刘耀宗 , 杨震 . 3-去羟基Phomonol的合成[J]. 有机化学, 2019 , 39(9) : 2671 -2675 . DOI: 10.6023/cjoc201903014
A concise synthesis of 3-dehydroxyphomonol has been accomplished in six steps from commercially available (R)-4-chloro-3-hydroxy-butyric acid ethyl ester or (S)-2-propyl-oxirane. The key steps involved Grignard reaction, Prins cyclization and palladium-catalyzed intramolecular alkoxycarbonylation to install the tetrahydropyran ring. The synthesis demonstrated an application of protecting-group-free strategy.

[1] (a) Wright, A. E.; Botelho, J. C.; Guzman, E.; Harmody, D.; Linley, P.; McCarthy, P. J.; Pitts, T. P.; Pomponi, S. A.; Reed, J. K. J. Nat. Prod. 2007, 70, 412.
(b) Yu, J. F.; Feng, R. K.; Yang, Z. Chin. J. Org. Chem. 2017, 37, 2526(in Chinese).
[2] Pereira, A. R.; McCue, C. F.; Gerwick, W. H. J. Nat. Prod. 2010, 73,.
[3] Kito, K.; Ookura, R.; Yoshida, S.; Namikoshi, M.; Ooi, T.; Kusumi, T. Org. Lett. 2008, 10, 225.
[4] Sikorska, J.; Hau, A. M.; Anklin, C.; Parker-Nance, S.; Da-vies-C, M. T.; Ishmael, J. E.; McPhail, K. L. J. Org. Chem. 2012, 77, 6066.
[5] Han, X.; Peh, G. R.; Floreancig, P. E. Eur. J. Org. Chem. 2013, 1193.
[6] Hua, J. L.; Bian, M.; Ding, H. F. Tetrahedron Lett. 2016, 57, 5519.
[7] Romero, K. J.; Galliher, M. S.; Pratt, D. A.; Stephenson-Corey, R. J. Chem. Soc. Rev. 2018, 47, 7851.
[8] Bai, Y.; Davis, D. C.; Dai, M. J. J. Org. Chem. 2017, 82, 2319.
[9] Heravi, M. M.; Ahmadi, T.; Ghavidel, M.; Heidari, B.; Hamidi, H.; RSC Adv. 2015, 5, 101999.
[10] Li Y. Y.; Wang, M. Z.; Huang, Y. J.; Shen, Y. M. Mycology 2010, 254.
[11] (a) Krishna, P. R.; Prabhakar, S. Tetrahedron Lett. 2013, 54, 3788.
(b) Reddy, B-V. S.; Srinivas, L. P.; Reddy, S.; Reddy, B. P.; Prasad, A. R.; Yadav, J. S. Helv. Chim. Acta 2014, 97, 1326.
(c) Bhaskar. K.; Jangilib, P.; Anireddy, J. S. J. Pharm. Res. 2015, 9, 550.
(d) Garrido, F.; Santalla, H.; Góme, G.; Fall, Y. Tetrahedron Lett. 2016, 57, 2631.
(e) Gahalawat, S.; Pandey, S. K. Tetrahedron Lett. 2017, 58, 2898.
(f) Wang, X.; Feng, R. K.; Qi, C. Z.; Yang, Z. Tetrahedron Lett. 2017, 58, 3588.
[12] ?asar, Z. Synlett 2008, 2036.
[13] LarchevGque, M.; Henrot, S. Tetrahedron 1990, 46, 4277.
[14] Yang, Z.; Xie, X.; Jing, P.; Zhao, G.; Zheng, J.; Zhao, C.; She, X. Org. Biomol. Chem. 2011, 9, 984.
/
| 〈 |
|
〉 |