

含三氟甲基的2,4-取代嘧啶衍生物的合成及抗肿瘤活性评价
收稿日期: 2019-03-13
修回日期: 2019-04-16
网络出版日期: 2019-04-26
基金资助
国家自然科学基金(No.81430085)、河南省自然科学基金(No.182300410321)资助项目.
Synthesis and Antitumor Activity Evaluation of 2,4-Substituted Py-rimidine Derivatives Containing Trifluoromethyl
Received date: 2019-03-13
Revised date: 2019-04-16
Online published: 2019-04-26
Supported by
Project supported by the National Natural Science Foundation of China (No. 81430085) and the Natural Science Foundation of Henan Province (No. 182300410321).
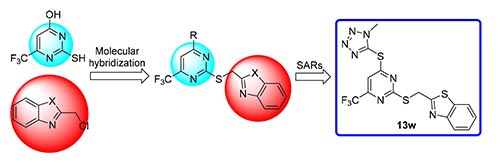
为了寻找高效的新型抗肿瘤药物,设计并合成了一系列新型含三氟甲基的2,4-取代嘧啶衍生物,并对目标化合物在EC-109(人食管癌细胞),MGC-803(人胃癌细胞),PC-3(人前列腺癌细胞)和HepG-2(人肝癌细胞)进行抗肿瘤活性评价,结果显示部分化合物对PC-3表现出中度至强效的抗肿瘤活性.其中,2-(((4-((1-甲基-1H-四唑-5-基)硫基)-6-(三氟甲基)嘧啶-2-基)硫基)甲基)苯并[d]噻唑(13w)对PC-3具有较强的抗肿瘤活性,IC50为1.76 μmol·L-1,抗肿瘤活性明显优于阳性对照药5-氟尿嘧啶.
关键词: 三氟甲基; 2,4-取代嘧啶衍生物; 抗肿瘤活性
孟娅琪 , 李二冬 , 张洋 , 刘栓 , 包崇男 , 杨鹏 , 张路野 , 张丹青 , 王继宽 , 陈雅欣 , 栗娜 , 辛景超 , 赵培荣 , 可钰 , 张秋荣 , 刘宏民 . 含三氟甲基的2,4-取代嘧啶衍生物的合成及抗肿瘤活性评价[J]. 有机化学, 2019 , 39(9) : 2541 -2548 . DOI: 10.6023/cjoc201903022
In order to find more effective antitumor drugs, a series of novel 2,4-substituted pyrimidine derivatives containing trifluoromethyl were designed, synthesized, and evaluated for antitumor activity aganist EC-109 (human esophageal cancer cell), MGC-803 (human gastric cancer cell), PC-3 (human prostate cancer cell) and HepG-2 (human liver cancer cell). The results showed that some compounds displayed moderate to potent antitumor activity against PC-3. Among them, 2-(((4-((1-methyl-1H-tetrazol-5-yl)thio)-6-(trifluoromethyl)pyrimidin-2-yl)thio)methyl)benzo[d]thiazole (13w) possesses strong antitu-mor activity against PC-3 with IC50 value of 1.76 μmol·L-1, and the antitumor activity is significantly better than the positive control drug of 5-fluorouracil.

[1] Addepalli, Y.; Yang, X.; Zhou, M.; Reddy, D. P.; Zhang, S. L.; Wang, Z.; He, Y. Eur. J. Med. Chem. 2018, 151, 214.
[2] Akhtar, J.; Khan, A. A.; Ali, Z.; Haider, R.; Shahar Yar, M. Eur. J. Med. Chem. 2017, 125, 143.
[3] Okesli, A.; Khosla, C.; Bassik, M. C. Curr. Opin. Biotechnol. 2017, 48, 127.
[4] Kahriman, N.; Serdaroglu, V.; Peker, K.; Aydin, A.; Usta, A.; Fandakli, S.; Yayli, N. Bioorg. Chem. 2019, 83, 580.
[5] Tageldin, G. N.; Fahmy, S. M.; Ashour, H. M.; Khalil, M. A.; Nassra, R. A.; Labouta, I. M. Bioorg. Chem. 2018, 78, 358.
[6] Li, Z. H.; Liu, X. Q.; Zhao, T. Q.; Geng, P. F.; Guo, W. G.; Yu, B.; Liu, H. M. Eur. J. Med. Chem. 2017, 139, 741.
[7] Abdellatif, K. R. A.; Bakr, R. B. Bioorg. Chem. 2018, 78, 341.
[8] Kuppast, B.; Fahmy, H. Eur. J. Med. Chem. 2016, 113, 198.
[9] Zhang, H.; Wang, J.; Shen, Y.; Wang, H. Y.; Duan, W. M.; Zhao, H. Y.; Hei, Y. Y.; Xin, M.; Cao, Y. X.; Zhang, S. Q. Eur. J. Med. Chem. 2018, 148, 221.
[10] Jabbour, E. Am. J. Hematol. 2016, 91, 59
[11] Usachev, B. I. J. Fluorine Chem. 2018, 210, 6.
[12] Kaur, K.; Kumar, V.; Gupta, G. K. J. Fluorine Chem. 2015, 178, 306.
[13] Xin, M.; Zhang, L.; Wen, J.; Shen, H.; Liu, Z.; Zhao, X.; Jin, Q.; Wang, M.; Cheng, L.; Huang, W.; Tang, F. Bioorg. Med. Chem. 2016, 24, 1079.
[14] Carmine, A. A.; Broden R. N.; Heel, R. C.; Speight, T. M.; Avery, G. S. Drugs 1982, 23, 329.
[15] Xie, X.; Yan, Y.; Zhu, N.; Liu, G. Eur. J. Med. Chem. 2014, 76, 67.
[16] Abdelgawad, M. A.; Bakr, R. B.; Omar, H. A. Bioorg. Chem. 2017, 74, 82.
[17] Akhtar, W.; Khan, M. F.; Verma, G.; Shaquiquzzaman, M.; Rizvi, M. A.; Mehdi, S. H.; Akhter, M.; Alam, M. M. Eur. J. Med. Chem. 2017, 126, 705.
[18] Li, N.; Xin, J. C.; Meng, Y, Q.; Li, E, D.; Ma, Q, S.; Bao, C, N.; Yang, P.; Song, P, P.; Cui, F.; Zhao, P, R.; Li, W.; Ke, Y.; Zhang, Q, R.; Liu, H, M. Chin. J. Org. Chem. 2018, 38, 2673.
[19] Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P. M.; Dhar, K. L. Eur. J. Med. Chem. 2014, 77, 422.
[20] Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Eur. J. Med. Chem. 2017, 142, 179.
[21] Lokwani, D.; Azad, R.; Sarkate, A.; Reddanma, P.; Shinde, D. Bioorg. Med. Chem. 2015, 23, 4533.
[22] Chen, P. J.; Yang, A.; Gu, Y. F.; Zhang, X. S.; Shao, K. P.; Xue, D. Q.; He, P.; Jiang, T. F.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 2741.
[23] Hao, Y.; Chen, Y. Dyes Pigm. 2016, 129, 186.
[24] Gellis, A.; Boufatah, N.; Vanelle, P. Green Chem. 2006, 8, 483.
/
| 〈 |
|
〉 |