

Pd(OAc)2/N-氯-N-氟苯磺酰胺体系下乙酰苯胺的氯化反应
收稿日期: 2019-03-20
修回日期: 2019-04-25
网络出版日期: 2019-05-10
基金资助
国家自然科学基金(No.21372077)和低品位磷矿高效利用国家重点实验室(No.WFKF2017-04)资助项目.
Chlorination of Anilide by Pd(OAc)2/N-Chloro-N-fluorobenzene- sulfonylamide
Received date: 2019-03-20
Revised date: 2019-04-25
Online published: 2019-05-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372077) and the State Key Laboratory of Efficient Utilization for Low Grade Phosphate Rock and Its Associated Resources (No. WFKF2017-04).
朱晔 , 黄金文 , 杨先金 . Pd(OAc)2/N-氯-N-氟苯磺酰胺体系下乙酰苯胺的氯化反应[J]. 有机化学, 2019 , 39(6) : 1665 -1671 . DOI: 10.6023/cjoc201903037
A mild method for palladium-catalyzed halogenation of acetanilide with N-chloro-N-fluorobenzenesulfonylamide (CFBSA) as a chlorinating reagent, oxidant, and novel promoting reagent was achieved. The decomposition of byproduct N-fluoroben-zenesulfonylamine in the presence of Pd(OAc)2 could accelerate the process of chlorination. Preliminary mechanism investigation showed that Pd catalyzed anilide directed C-H activation lead to the ortho chlorination selectivity. A series of ortho-chlorinated anilides were obtained in 28%~82% yields.
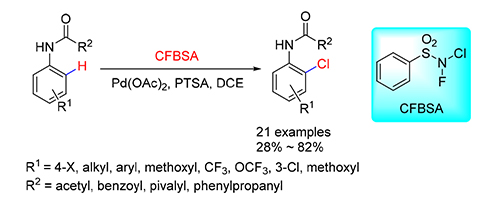
Key words: chlorinating reagent; fluorine atom; Pd-catalyzed; synthetic methodology
[1] (a) Kinsinger, T.; Kazmaier, U. Org. Lett. 2018, 20, 7726.
(b) Nasrollahzadeh, M.; Issaabadi, Z.; Tohidi, M. M.; Sajadi, S. M. Chem. Rev. 2018, 18, 165.
(c) Ding, H.; Li, J.; Guo, Q.; Xiao, Y. Chin. J. Org. Chem. 2017, 37, 3112.
(d) Wang, J.; Li, F.; Yu, X.; Liu, L.; Ding, J.; Xie, P.; Wang, J. Chin. J. Org. Chem. 2018, 38, 1638.
(e) Davie, E. A. C.; Mennen, S. M.; Xu, Y.; Miller, S. J. Chem. Rev. 2007, 107, 5759.
[2] (a) Seifert, S.; Schmidt, D.; Shoyama, K.; Wuerthner, F. Angew. Chem., Int. Ed. 2017, 56, 7595.
(b) Li, H.; Shi, Z.-J. Prog. Chem. 2010, 22, 1414.
(c) Liang, J.-Y.; Shen, S.-J.; Xu, X.-H.; Fu, Y.-L. Org. Lett. 2018, 20, 6627.
(d) Manikandan, T. S.; Ramesh, R.; Semeril, D. Organometallics 2019, 38, 319.
(e) Wang, Y.; Zeng, J.; Cui, X. Chin. J. Org. Chem. 2010, 30, 181.
[3] (a) Sehnal, P.; Taylor, R. J. K.; Fairlamb, I. J. S. Chem. Rev. 2010, 110, 824.
(b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147.
[4] (a) Dilauro, G.; Quivelli, A. F.; Vitale, P.; Capriati, V.; Perna, F. M. Angew. Chem., Int. Ed. 2019, 58, 1799.
(b) Youn, S. W.; Kim, Y. H.; Jo, Y. H. Adv. Synth. Catal. 2019, 361, 462.
[5] (a) Mei, C.; Lu, W. J. Org. Chem. 2018, 83, 4812.
(b) Wang, G.-W.; Yuan, T.-T.; Wu, X.-L. J. Org. Chem. 2008, 73, 4717.
(c) Karthikeyan, J.; Cheng, C.-H. Angew. Chem., Int. Ed. 2011, 50, 9880.
[6] (a) Gao, X.-A.; Yan, R.-L.; Wang, X.-X.; Yan, H.; Li, J.; Guo, H.; Huang, G.-S. J. Org. Chem. 2012, 77, 7700.
(b) Xing, X.; O'Connor, N. R.; Stoltz, B. M. Angew. Chem., Int. Ed. 2015, 54, 11186.
(c) Saito, F.; Aiso, H.; Kochi, T.; Kakiuchi, F. Organometallics 2014, 33, 6704.
[7] (a) Lu, O.; Huang, J.; Li, J.; Qi, C.; Wu, W.; Jiang, H. Chem. Commun. 2017, 53, 10422.
(b) Khatun, N.; Modi, A.; Ali, W.; Patel, B. K. J. Org. Chem. 2015, 80, 9662.
[8] Ma, C.; Zhao, C.-Q.; Li, Y.-Q.; Zhang, L.-P.; Xu, X.-T.; Zhang, K.; Mei, T.-S. Chem. Commun. 2017, 53, 12189.
[9] Wang, X.; Leow, D.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 13864.
[10] (a) Dong, Y.; Liu, G. J. Org. Chem. 2017, 82, 3864.
(b) Jin, L.; Zeng, X.; Li, S.; Hong, X.; Qiu, G.; Liu, P. Chem. Commun. 2017, 53, 3986.
[11] (a) Chen, C.-H.; Luo, Y.-X.; Fu, L.; Chen, P.-H.; Lan, Y.; Liu, G.-S. J. Am. Chem. Soc. 2018, 140, 1207.
(b) Chen, C.-H.; Chen, P.-H.; Liu, G.-S. J. Am. Chem. Soc. 2015, 137, 15648.
[12] Liu, R.; Lu, Z.-H.; Hu, X.-H.; Li, J.-L.; Yang, X.-J. Org. Lett. 2015, 17, 1489.
[13] Lu, Z.-H.; Li, Q.-W.; Tang, M.-H.; Jiang, P.-P.; Zheng, H.; Yang, X.-J. Chem. Commun. 2015, 51, 14852.
[14] (a) Kim, K.; Jung, Y.; Lee, S.; Kim, M., Shin, D.; Byun, H.; Cho, S. J.; Song, H.; Kim, H. Angew. Chem., Int. Ed. 2017, 56, 6952.
(b) Bedford, R. B.; Haddow, M. F.; Mitchell, C. J.; Webster, R. L. Angew. Chem., Int. Ed. 2011, 50, 5524.
[15] Wan, X.-B.; Ma, Z.-X.; Li, B.-J.; Zhang, K.-Y.; Cao, S.-K.; Zhang, S.-W.; Shi, Z.-J. J. Am. Chem. Soc. 2006, 128, 7416.
[16] Lengyel, I.; Cesare, V.; Stephani, R. Synth. Commun. 1998, 28, 1891.
[17] Xiong, X. D.; Yeung, Y. Y. Angew. Chem., Int. Ed. 2016, 55, 16101.
[18] Hering, T.; Muehldorf, B.; Wolf, R.; Koenig, B. Angew. Chem., Int. Ed. 2016, 55, 5342.
[19] Bedford, R. B.; Engelhart, J. U.; Haddow, M. F.; Mitchell, C. J.; Webster, R. L. Dalton Trans. 2010, 39, 10464.
[20] Singh, H.; Sen, C.; Sahoo, T.; Ghosh, S. C. Eur. J. Org. Chem. 2018, 4748.
[21] Pu, X.-Q.; Zhao, H.-Y.; Lu, Z.-H.; He, X.-P.; Yang, X.-J. Eur. J. Org. Chem. 2016, 4526.
[22] Pu, X.-Q.; Li, Q.-W.; Lu, Z.-H.; Yang, X.-J. Eur. J. Org. Chem., 2016, 5937.
[23] Zhao, H.-Y.; Pu, X.-Q.; Yang, X.-J. Chin. J. Chem. 2017, 35, 1417.
/
| 〈 |
|
〉 |