

两亲聚肽/螺烯的电荷复合自组装行为研究
收稿日期: 2019-04-15
修回日期: 2019-05-07
网络出版日期: 2019-05-21
基金资助
国家自然科学基金(No. 51573088)
Study of Charge-Conjugated Self-Assembly Behavior of Amphiphilic Block Copolypeptides/Helicene
Received date: 2019-04-15
Revised date: 2019-05-07
Online published: 2019-05-21
Supported by
Project supported by the National Natural Science Foundation of China(No. 51573088)
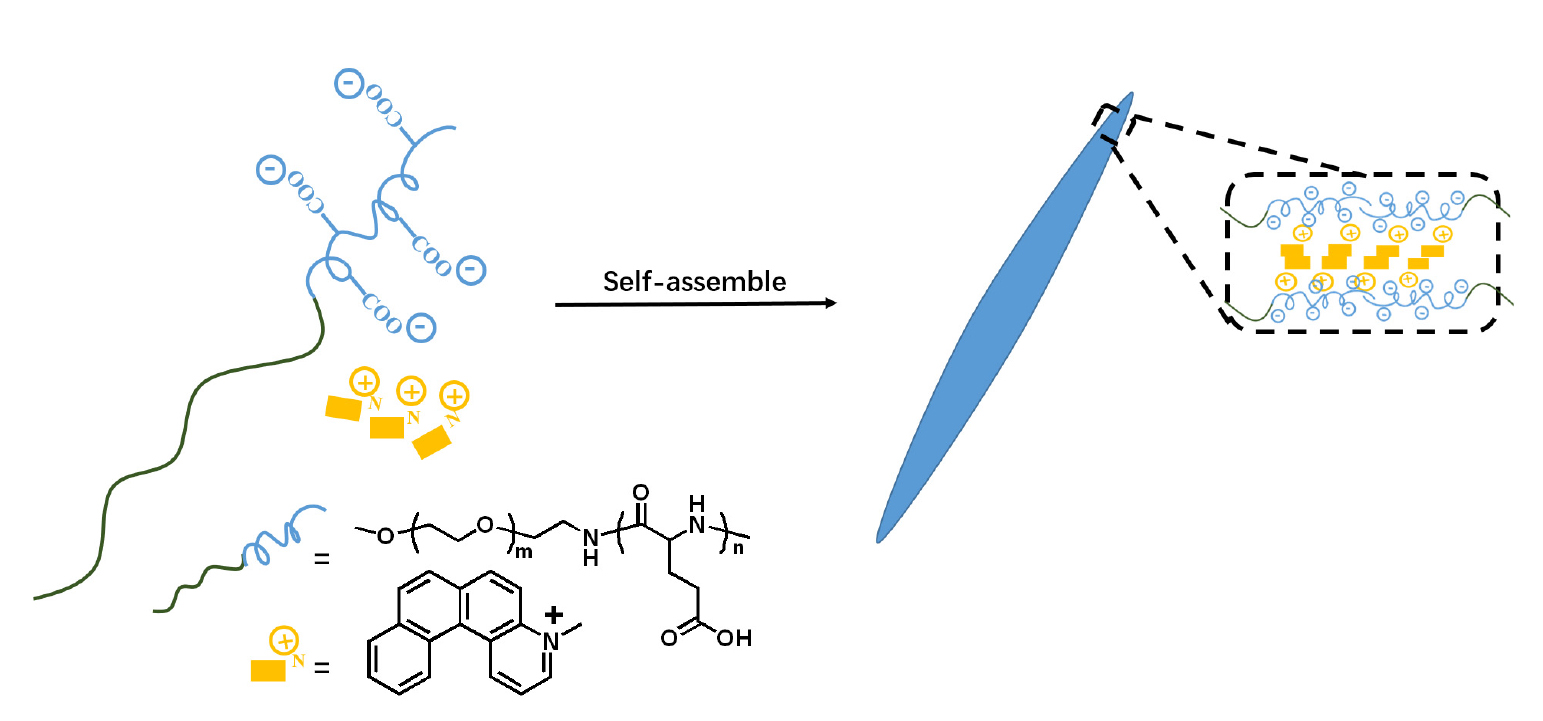
研究了包含聚肽嵌段的两亲共聚物聚乙二醇-b-聚(L-谷氨酸)(PEG-b-PGlu)与4-甲基-4-氮杂[4]螺烯鎓离子(Me[4]H)的电荷复合自组装行为, 讨论了聚肽链段长度、谷氨酸单元与螺烯物质的量比、pH值等因素对组装体形貌的影响. 研究发现, 当刚-柔型嵌段聚合物与刚性、大尺寸并具有诱导作用的小分子共同组装时, 遵循与柔性大分子电荷复合组装完全不同的规律. PEG-b-PGlu/Me[4]H的电荷复合组装体形貌由π-π堆叠作用、两亲嵌段聚肽的亲疏水比、PGlu链段的刚硬程度共同决定, 这些作用在组装过程中相互制约, 当π-π堆叠作用占主导地位时, 得到以指针状和条带状等平面结构为主的组装形貌.
王婧琳 , 沈程硕 , 唐颂超 , 姚远 . 两亲聚肽/螺烯的电荷复合自组装行为研究[J]. 有机化学, 2019 , 39(10) : 2973 -2979 . DOI: 10.6023/cjoc201904038
Amphiphilic block copolypeptides of polyethylene glycol-b-poly(L-glutamic acid) (PEG-b-PGlu) were synthesized to self-assemble with 4-methyl-4-aza[4]helicene onium ion (Me[4]H) in water through charge-conjugation. The morphology of the assemblies was studied by varying PGlu block length, the molar ratio of Glu unit/Me[4]H and pH value. It was found that when rigid-flexible block copolymers were assembled together with small molecules which had rigid, large size and inductive effect, they would follow a completely different law from the charge-conjugated assembly of flexible macromolecules. The self-assemblies were controlled by the π-π stacking of helicene, together with the volume fraction of the hydrophobic parts and the rigidity of PGlu segments. These effects restricted each other in the assembly process. When π-π stacking was dominant, assembly morphologies with pointer-like and strip-like planar structures were obtained.

| [1] | Gr?hn, F. Soft Matter 2010, 6, 4296. |
| [2] | Rybtchinski, B. ACS Nano 2011, 5, 6791. |
| [3] | Faul, C. F. Acc. Chem. Res. 2014, 47, 3428. |
| [4] | Kim, B. G.; Kim, M. S.; Kim, J . ACS Nano 2010, 4, 2160. |
| [5] | Malinsky, J. E.; Jabbour, G. E.; Shaheen, S. E.; Anderson, J. D.; Richter, A. G.; Marks, T. J.; Armstrong, N. R.; Kippelen, B.; Dutta, P.; Peyghambarian, N . Adv. Mater. 1999, 11, 227. |
| [6] | Palma, A.; Satta, M. J. Chem. Theory Comput. 2016, 12, 4042. |
| [7] | Li, Y.; Zhang, X.; Cao, D. J. Phys. Chem. B 2013, 117, 6733. |
| [8] | Zhao, Y.; Mei, L.; Lu, Q . Langmuir 2008, 24, 3937. |
| [9] | Wicklein, A.; Ghosh, S.; Sommer, M.; Würthner, F.; Thelakkat, M . ACS Nano 2009, 3, 1107. |
| [10] | Wang, C.; Guo, Y.; Wang, Z.; Zhang, X . Langmuir 2010, 26, 14509. |
| [11] | Yao, Y.; Zhang, L.; Leydecker, T.; Samorì, P. J. Am. Chem. Soc. 2018, 140, 6984. |
| [12] | Liu, W.; Liu, J.; Liu, W.; Li, T. J. Agric. Food Chem. 2013, 61, 4133. |
| [13] | Omura, Y.; Kyung, K. H.; Shiratori, S.; Kim, S. H. Ind. Eng. Chem. Res. 2014, 53, 11727. |
| [14] | Fang, R.; Zhang, H.; Yang, L.; Wang, H.; Tian, Y.; Zhang, X.; Jiang, L. J. Am. Chem. Soc. 2016, 138, 16372. |
| [15] | Pappa, A. M.; Inal, S.; Roy, K.; Zhang, Y. ACS Appl. Mater. Inter. 2017, 9, 10427. |
| [16] | Dochter, A.; Garnier, T.; Pardieu, E.; Chau, N. T. T.; Maerten, C.; Senger, B.; Schaaf, P.; Jierry, L.; Boulmedais, F . Langmuir 2015, 31, 10208. |
| [17] | Bianchi, R. C.; Silva, E. R. D.; Antonia, L. H. D.; Ferreira, F. F.; Alves, A. W . Langmuir 2014, 30, 11464. |
| [18] | Deng, T.; Wang, J.; Li, Y. Y.; Han, Z. H.; Peng, Y. N.; Zhang, J.; Gao, Z.; Gu, Y. Q.; Deng, D. W. ACS Appl. Mater. Interfaces 2018, 10, 27657. |
| [19] | Huang, X. H.; Jeong, Y. I.; Moon, B. K.; Zhang, L. D.; Kang, D. H.; Kim, I . Langmuir 2013, 29, 3223. |
| [20] | Bui, L.; Abbou, S.; Ibarboure, E.; Guidolin, N.; Staedel, C.; Toulme, J. J.; Schatz, C. J. Am. Chem. Soc. 2012, 134, 20189. |
| [21] | Kumar, R. J.; Macdonald, J. M.; Singh, T. B.; Waddington, L. J.; Holmes, A. B. J. Am. Chem. Soc. 2011, 133, 8564. |
| [22] | Zhang, Y.; Yin, Q.; Lu, H.; Xia, H.; Lin, Y.; Cheng, J. ACS Macro Lett. 2013, 2, 809. |
| [23] | Shaikh, A. Y.; Das, S.; Pati, D.; Dhaware, V.; Sen, G. S.; Hotha, S . Biomacromolecules 2014, 15, 3679. |
| [24] | Ramasamy, T.; Choi, J. Y.; Cho, H. J.; Umadevi, S. K.; Shin, B. S.; Choi, H. G.; Yong, C. S.; Kin, J. O . Pharm. Res. 2015, 32, 1947. |
| [25] | He, W.; Yan, J.; Jiang, W.; Li, S.; Qu, Y.; Niu, F.; Yan, Y.; Sui, F.; Wang, S.; Zhou, Y.; Jin, L.; Li, Y.; Ji, M.; Ma, P. X.; Liu, W.; Hou, P . Chem. Mater. 2018, 30, 7034. |
| [26] | Yan, J.; Korolev, N.; Eom, K. D.; Tam, J. P.; Nordenski?ld, L . Biomacromolecules 2012, 13, 124. |
| [27] | Nishimura, T.; Yamada, A.; Umezaki, K.; Sawada, S. I.; Mukai, S. A.; Sasaki, Y.; Akiyoshi, K . Biomacromolecules 2017, 18, 3913. |
| [28] | Ryu, K.; Lee, M.; Park, J.; Kim, T. ACS Appl. Bio. Mater. 2018, 1, 1496. |
| [29] | Zhang, S.; Cai, C.-H.; Huang, Q.-J.; Lin, J.-P.; Xu, Z.-W . Acta Polym. Sin. 2018,109(in Chinese). |
| [29] | ( 张朔, 蔡春华, 黄琦婧, 林嘉平, 徐占文 , 高分子学报, 2018,109.) |
| [30] | Hu, Y.; Lin, R.; Zhang, P.; Fern, J.; Cheetham, A. G.; Patel, K.; Schulman, R.; Kan, C.; Cui, H . ACS Nano 2015, 10, 880. |
| [31] | Schuster, N. J.; Hernández, S. R.; Bukharina, D.; Kotov, N. A.; Berova, N.; Nq, F.; Steiqerwald, M. L.; Nuckolls, C. J. Am. Chem. Soc. 2018, 140, 6235. |
| [32] | Nishiyma, N.; Okazaki, S.; Cabral, H.; Miyamto, M.; Kato, Y.; Suqiyama, Y.; Nishio, K.; Matsumura, Y.; Kataoka, K . Cancer Res. 2003, 63, 8977. |
| [33] | Lv, S.; Li, M.; Tang, Z.; Song, W.; Sun, H.; Liu, H.; Chen, X . Acta Biomater. 2013, 9, 9330. |
| [34] | Song, W.; Tang, Z.; Li, M.; Lv, M.; Sun, H.; Deng, M.; Liu, H.; Chen, X . Acta Biomater. 2014, 10, 1392. |
| [35] | Verbiest, T.; Elshocht, S. V.; Kauranen, M.; Hellemans, L.; Snauwaert, J.; Nuckolls, C.; Katz, T. J.; Persoons, A . Science 1999, 561, 913. |
| [36] | Fang, L.; Lin, W.-B.; Shen, Y.; Chen, C.-F. Chin. J. Org. Chem. 2018, 38, 541 (in Chinese). |
| [36] | ( 房蕾, 林伟彬, 沈赟, 陈传峰, 有机化学, 2018, 38, 541.) |
| [37] | Kaseyama, T.; Furumi, S.; Zhang, X.; Takeuchi, M. Angew. Chem., Int. Ed. 2011, 123, 3768. |
| [38] | Hewlins, M, J, E.; Salter, R . Synthesis 2007,2164. |
| [39] | Yao, Y.; Li, W. W.; Wang, S. B.; Yan, D. Y.; Chen, X. S . Macromol. Rapid Commun. 2006, 27, 2019. |
/
| 〈 |
|
〉 |