

脱氢偶联制备芳基醚方法的研究进展
收稿日期: 2019-04-04
修回日期: 2019-05-09
网络出版日期: 2019-06-06
基金资助
浙江省自然科学基金(LQ19B020002);宁波市自然科学基金(2018A610241);浙江省教育厅(Y201839228);宁波大学王宽诚幸福基金资助项
Advances on the Synthesis of Aryl Ethers via Dehydrogenative Coupling
Received date: 2019-04-04
Revised date: 2019-05-09
Online published: 2019-06-06
Supported by
Project supported by the Natural Science Foundation of Zhejiang Province(LQ19B020002);The Municipal Natural Science Foundation of Ningbo City(2018A610241);The Education Foundation of Zhejiang Province(Y201839228);The K. C. Wong Magna Fund in Ningbo University
唐灏 , 骆钧飞 , 解攀 . 脱氢偶联制备芳基醚方法的研究进展[J]. 有机化学, 2019 , 39(10) : 2735 -2743 . DOI: 10.6023/cjoc201904011
Aryl ethers are important central motifs that are abundant in many natural products and drug molecules, as well as versatile building blocks for organic synthesis. Aryl ethers were usually synthesized through the coupling reactions between leaving group substituted arenes and alcohols. However, the introduction of leaving group requires extra synthetic operation and produces lots of wastes. Over the past decade, the method for the synthesis of aryl ethers via C—H alkoxylation or aryloxylation has received much attention due to its potential as an atom and step efficient methodology. Herein, the research advances on the synthesis of aryl ethers through dehydrogenative coupling are reviewed. The detailed substrate scopes and reaction mechanisms, as well as the limitations of current procedures and the prospects for the future, are discussed.
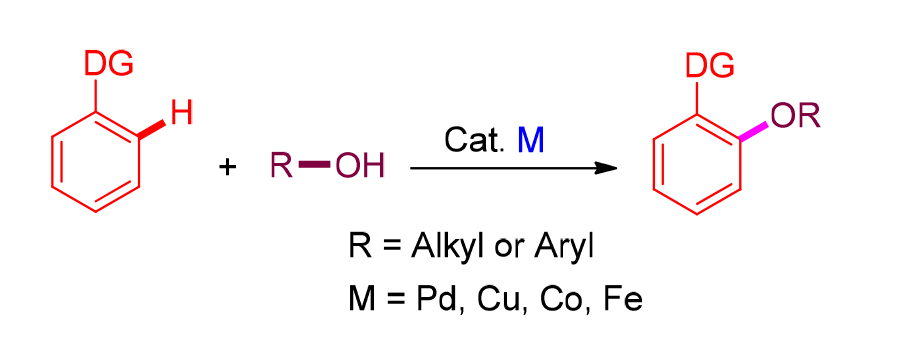
Key words: aryl ether; dehydrogenative coupling; C—H activation; phenol; alcohol
| [1] | (a) Jabran, K.; Ehsanullah; Hussain, M.; Farooq, M.; Babar, M.; Do?an, M.-N.; Lee, D.-J. Weed Biol. Manage. 2012, 12, 136. |
| [1] | (b) Negro, R.; Formoso, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. J. Clin. Endocrinol. Metab. 2006, 91, 2587. |
| [1] | (c) Kosenkova, Y.-S.; Polovinka, M.; Komarova, N.; Korchagina, D.; Kurochkina, N. Y.; Cheremushkina, V.; Salakhutdinov, N. Chem. Nat. Compd. 2007, 43, 712. |
| [1] | (d) Deng, H.; Jung, J.-K.; Liu, T.; Kuntz, K. W.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 9032. |
| [2] | (a) Chen, G.; Du, J. Chin. J. Org. Chem. 2014, 34, 65 (in Chinese). |
| [2] | ( 陈国军, 杜建时, 有机化学, 2014, 34, 65 ) |
| [2] | (b) Tobisu, M.; Chatani, N. Acc. Chem. Res. 2015, 48, 1717. |
| [3] | (a) Ullmann, F.; Sponagel, P. Ber. Dtsch. Chem. Ges. 1905, 38, 2211. |
| [3] | (b) Xiao, S.; Zhu, J.; Mu, X.; Li, Z. Chin. J. Org. Chem. 2013, 33, 1668 (in Chinese). |
| [3] | ( 肖尚友, 朱俊, 穆小静, 李正华, 有机化学, 2013, 33, 1668.) |
| [4] | (a) Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933. |
| [4] | (b) Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937. |
| [4] | (c) Lam, P. Y. S.; Vincent, G.; Clark, C. G.; Deudon, S.; Jadhav, P. K. Tetrahedron Lett. 2001, 42, 3415. |
| [5] | (a) Aranyos, A.; Old, D. W.; Kiyomori, A.; Wolfe, J. P.; Sadighi, J. P.; Buchwald, S. L. J. Am. Chem. Soc. 1999, 121, 4369. |
| [5] | (b) Torraca, K. E.; Huang, X.; Parrish, C. A.; Buchwald, S. L. J. Am. Chem. Soc. 2001, 123, 10770. |
| [5] | (c) Mann, G.; Hartwig, J. F. J. Am. Chem. Soc. 1996, 118, 13109. |
| [6] | Terrett, J. A.; Cuthbertson, J. D.; Shurtleff, V. W MacMillan, D. W. C .; Nature 2015, 524, 330. |
| [7] | (a) Yang, F.; Zhang, H.; Liu, X.; Wang, B.; Ackermann, L. Chin. J. Org. Chem. 2019, 39, 59 (in Chinese). |
| [7] | ( 杨帆致, 张晗, 刘旭日, 王博, Lutz, A., 有机化学, 2019, 39, 59) |
| [7] | (b) Leila, R.; Asieh, Y.; Mehdi, S. ChemCatChem 2018, 10, 20. |
| [7] | (c) Kamellia, N.; Sheida, A.; Mohammad, N.; Parvaneh, D. K. N.; Esmail, V. RSC Adv. 2018, 8, 19125 |
| [7] | (d) Bhunia, S.; Pawar, G. G.; Kumar, S. V.; Jiang, Y.; Ma, D. Angew. Chem., Int. Ed. 2017, 56, 16136 |
| [7] | (e) Ding, H.; Li, J.; Guo, Q.; Xiao, Y. Chin. J. Org. Chem.(in Chinese). 2017, 37, 3112 |
| [7] | ( 丁怀伟, 李娟, 郭庆辉, 肖琰, 有机化学, 2017, 37, 3112) |
| [8] | Desai, L. V.; Malik, H. A.; Sanford, M. S. Org. Lett. 2006, 8, 1141. |
| [9] | Wang, G.-W.; Yuan, T.-T. J. Org. Chem. 2010, 75, 476. |
| [10] | Jiang, T.-S.; Wang, G.-W. J. Org. Chem. 2012, 77, 9504 |
| [11] | Shi, S.-P.; Kuang, C.-X. J. Org. Chem. 2014, 79, 6105. |
| [12] | Li, W.; Sun, P.-P. J. Org. Chem. 2012, 77, 8362 |
| [13] | Yin, Z.-W.; Jiang, X.-Q.; Sun, P.-P. J. Org. Chem. 2013, 78, 10002 |
| [14] | Zhang, C .; Sun, P.-P. J. Org. Chem. 2014, 79, 8457. |
| [15] | Gao, T.-T.; Sun, P.-P. J. Org. Chem. 2014, 79, 9888. |
| [16] | Peron, F.; Fossey, C.; Santos, J. O. S.; Cailly, T.; Fabis, F. Chem.- Eur. J. 2014, 20, 1. |
| [17] | Chen, F.-J.; Zhao, S.; Hu, F.; Chen, K.; Zhang, Q.; Zhang, S.-Q.; Shi, B.-F. Chem. Sci. 2013, 4, 4187. |
| [18] | (a) Shen, T.; Wang, X.-N.; Lou, H.-X. Nat. Prod. Rep. 2009, 26, 916. |
| [18] | (b) Veitch, N. C. Nat. Prod. Rep. 2007, 24, 417. |
| [18] | (c) Watzke, A.; O'Malley, S. J.; Bergman, R. G.; Ellman, J. A. J. Nat. Prod. 2006, 69, 1231. |
| [18] | (d) Tsui, G. C.; Tsoung, J.; Dougan, P.; Lautens, M. Org. Lett. 2012, 14, 5542. |
| [19] | Wang, X.-S.; Lu, Y.; Dai, H.-X.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 12203. |
| [20] | Wang, H.-B.; Li, G.; Engle, K. M.; Yu, J.-Q.; Davies, H. M. L. J. Am. Chem. Soc. 2013, 135, 6774. |
| [21] | Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S Yu, J.-Q .; J. Am. Chem. Soc. 2013, 135, 1236. |
| [22] | Wang, Z.-L.; Zhao, L.; Wang, M.-X. Org. Lett. 2011, 13, 6560. |
| [23] | Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, K.; Ren, B.-Z.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. J. Org. Chem. 2014, 79, 10399. |
| [24] | Bhadra, S.; Matheis, C.; Katayev, D.; Goo?en, L. J. Angew. Chem. 2013, 125, 9449. |
| [25] | Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842. |
| [26] | Yin, X.-S.; Li, Y.-C.; Yuan, J.; Gua, W.-J.; Shi, B.-F. Org. Chem. Front. 2015, 2, 119. |
| [27] | Zhang, L.-B.; Hao, X.-Q.; Zhang, S.-K.; Liu, Z.-J.; Zheng, X.-X.; Gong, J.-F.; Niu, J.-F.; Song, M.-P. Angew. Chem., Int. Ed. 2015, 54, 272. |
| [28] | Zheng, Y.-W.; Ye, P.; Chen, B.; Meng, Q.-Y.; Feng, K.; Wang, W.-G.; Wu, L.-Z.; Tung, C.-H. Org. Lett. 2017, 19, 2206. |
| [29] | Ohkubo, K.; Kobayashi, T.; Fukuzumi, S. Opt. Express 2012, 20, A360. |
| [30] | Tang, L.; Pang, Y.; Yan, Q.; Shi, L.-Q.; Huang, J.-H.; Du, Y.-F.; Zhao, K. J. Org. Chem. 2011, 76, 2744. |
| [31] | Xiao, B.; Gong, T.-J.; Liu, Z.-J.; Liu, J.-H.; Luo, D.-F.; Xu, J.; Liu, L. J. Am. Chem. Soc. 2011, 133, 9250. |
| [32] | Wei, Y.; Yoshikai, N. J. Org. Chem. 2011, 13, 5504. |
| [33] | Zhao, J.-J.; Wang, Y.; He, Y.-M.; Liu, L.-Y.; Zhu, Q. Org. Lett. 2012, 14, 1078. |
| [34] | (a) Zhao, J.-J.; Wang, Y.; Zhu, Q. Synthesis 2012, 44, 1551. |
| [34] | (b) Zhao, J.-J.; Zhang, Q.; Liu, L.-Y.; He, Y.-M.; Li, J.; Li, J.; Zhu, Q. Org. Lett. 2012, 14, 5362. |
| [35] | Roane, J.; Daugulis, O. Org. Lett. 2013, 15, 5842. |
| [36] | Hao, X.-Q.; Chen, L.-J.; Ren, B.; Li, L.-Y.; Yang, X.-Y.; Gong, J.-F.; Niu, J.-L.; Song, M.-P. Org. Lett. 2014, 16, 1104. |
| [37] | Zhou, Y.-F.; Zhu, J.-M.; Li, B.; Zhang, Y.; Feng, J.; Hall, A.; Shi, J.-Y.; Zhu, W.-L. Org. Lett.. 2016, 18, 3803 |
| [38] | Kumar, G. S.; Pieber, B.; Reddy, K. R.; Kappe, C. O. Chem.-Eur. J. 2012, 18, 6124. |
| [39] | Barve, B. D.; Wu, Y.-C.; El-Shazly, M.; Korinek, M.; Cheng, Y.-B.; Wang, J.-J.; Chang, F.-R. Tetrahedron 2015, 71, 2290 |
| [40] | Barve, B. D.; Wu, Y.-C El-Shazly, M.; Korinek, M.; Cheng, Y.-B.; Wang, J.-J.; Chang, F.-R. Org. Lett. 2014, 16, 1912. |
| [41] | Boominathan, S. S. K.; Hu, W.-P.; Senadi, G. C.; Vandavasia, J. K.; Wanga, J.-J. Chem. Commun. 2014, 50, 6726. |
/
| 〈 |
|
〉 |