

Cu(0)/Selectfluor体系催化的邻芳基磺酰亚胺的串联环化/芳构化反应:一种简便合成6H-菲啶的方法
收稿日期: 2019-04-24
修回日期: 2019-05-26
网络出版日期: 2019-06-12
基金资助
国家自然科学基金(21772176);国家自然科学基金(21372201);浙江工业大学“省重中之重一级学科”开放基金资助项目
Copper(0)/Selectfluor System-Catalyzed Tandem Annulation/Aromatization of o-Aryl Benzenesulfonylimides:A Facile Synthesis of 6H-Phenanthridines
Received date: 2019-04-24
Revised date: 2019-05-26
Online published: 2019-06-12
Supported by
Project supported by the National Natural Science Foundation of China(21772176);Project supported by the National Natural Science Foundation of China(21372201);The Opening Foundation of Zhejiang Key Course of Chemical Engineering and Technology, Zhejiang University of Technology
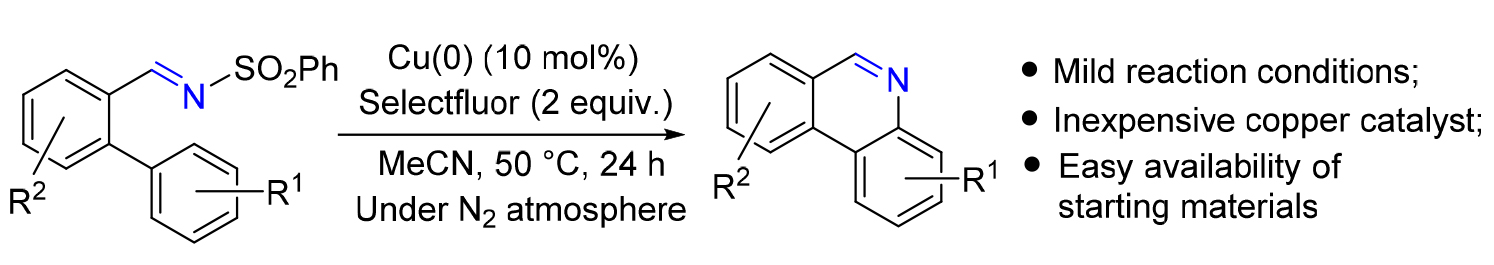
发展了Cu(0)/Selectfluor体系催化的邻芳基磺酰亚胺的串联环化/芳构化反应, 于温和的反应条件下以中等到良好的产率简便、高效地构建了一系列6H-菲啶类化合物. 机理研究表明, 反应的关键步骤经历了由Cu(0)/Selectfluor体系现场原位产生XCuOH (X=F, BF4)物种, 进而诱导对C=N键的羟铜化反应和分子内C—H键胺化反应, 从而合成了6H-菲啶类化合物.
关键词: 铜催化; Selectfluor; 6H-菲啶; 环化反应; C—H键胺化
郑立孟 , 施冬冬 , 鲍汉扬 , 刘运奎 . Cu(0)/Selectfluor体系催化的邻芳基磺酰亚胺的串联环化/芳构化反应:一种简便合成6H-菲啶的方法[J]. 有机化学, 2019 , 39(10) : 2821 -2828 . DOI: 10.6023/cjoc201904058
A facile and efficient method for the synthesis of 6H-phenanthridines has been successfully developed involving a copper(0)/Selectfluor system-catalyzed tandem annulation/aromatization of o-aryl benzenesulfonylimides. A variety of substituted 6H-phenanthridines were synthesized in moderate to good yields under mild reaction conditions. Mechanistic experiments revealed that the reaction might involve an oxycupration of C=N bond followed by an intramolecular C—H bond amination as the key steps triggered by an in situ generated copper species XCuOH (X=F or BF4) from the Cu(0)/Selectfluor system.

| [1] | (a) Cushman, M.; Mohan, P.; Smith, E.C. R. J. Med. Chem. 1984, 27, 544. |
| [1] | (b) Fang, S. D.; Wang, L. K.; Hecht, S.M. J. Org. Chem. 1993, 5025, 58. |
| [1] | (c) Lynch, M. A.; Duval, O.; Sukhanova, A.; Devy, J.; Mackay, S. P.; Waigh, R. D.; Nabiev, I. Bioorg. Med. Chem. Lett. 2001, 11, 2643. |
| [1] | (d) Bernardo, P. H.; Wan, K. F.; Sivaraman, T.; Xu, J.; Moore, F. K.; Hung, A. W.; Mok, H. Y. K.; Yu, V. C.; Chai, C. L. L. J. Med. Chem. 2008, 51, 6699. |
| [1] | (e) Zhu, S.; Ruchelman, A. L.; Zhou, N.; Liu, A.; Liu, L. F.; LaVoie, E.J. Bioorg. Med. Chem. 2005, 1, 6782. |
| [1] | (f) Li, D.; Zhao, B.; Sim, S.-P.; Li, T.-K.; Liu, A.; Liu, L. F.; Edmond, J.; LaVoie, E. J. Bioorg. Med. Chem. 2003, 11, 521. |
| [1] | (g) Tsukamoto, H.; Kondo, S.; Mukudai, Y.; Nagumo, T.; Yasuda, A.; Kurihara, Y.; Kamatani, T.; Shintani, S. Anticancer Res. 2011, 31, 2841. |
| [2] | (a) Cushman, M.; Mohan, P; Smith, E.C. R. . J. Med. Chem. 1984, 27, 544. |
| [2] | (b) Fang, S. D.; Wang, L. K.; Hecht, S.M. J. Org. Chem. 1993, 58, 5025. |
| [2] | (c) Lynch, M. A.; Duval, O.; Sukhanova, A.; Devy, J.; Mackay, S. P.; Waigh, R. D.; Nabiev, I. Bioorg. Med. Chem. Lett. 2001, 11, 2643. |
| [2] | (d) Bernardo, P. H.; Wan, K. F.; Sivaraman, T.; Xu, J.; Moore, F. K.; Hung, A. W.; Mok, H. Y. K.; Yu, V. C.; Chai, C. L. L. J. Med. Chem.2008, 51, 6699. |
| [2] | (e) Zhu, S.; Ruchelman, A. L.; Zhou, N.; Liu, A.; Liu, L. F.; LaVoie, E.J. Bioorg. Med. Chem. 2005, 13, 6782. |
| [2] | (f) Li, D.; Zhao, B.; Sim, S.-P.; Li, T.-K.; Liu, A.; Liu, L. F.; Edmond, J.; LaVoie, E.J. Bioorg. Med. Chem. 2003, 11, 521. |
| [2] | (g) Tsukamoto, H.; Kondo, S.; Mukudai, Y.; Nagumo, T.; Yasuda, A.; Kurihara, Y.; Kamatani, T.; Shintani, S. Anticancer Res. 2011, 31, 2841. |
| [3] | (a) Stevens, N.; O’Connor, N.; Vishwasrao, H.; Samaroo, D.; Kandel, E. R.; Akins, D. L.; Drain, C. M.; Rurro, N.J. J. Am. Chem. Soc. 2008, 130, 7182. |
| [3] | (b) Bondarev, S. L.; Knyukshto, V. N.; Tikhomirov, S. A.; Pyrko, A.N. Opt. Spectrosc. 2006, 100, 386. |
| [3] | (c) Zhang, J.; Lakowicz, J.R. J. Phys. Chem. B. 2005, 109, 8701. |
| [4] | For selected recent reviews, see: (a) Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505. |
| [4] | (b) Hayashi, H.; Kaga, A.; Chiba, S. J. Org. Chem. 2017, 82, 11981. |
| [4] | (c) Hu, B.; DiMagno, S.G. Org. Biomol. Chem. 2015, 13, 3844. |
| [5] | (a) Pictet, A.; Hubert, A. Ber. Dtsch. Chem. Ges. 1896, 29, 1182. |
| [5] | (b) Morgan, T.; Walls, L. P. J. Chem. Soc., 1931, 2447. |
| [5] | (c) Chinnagolla, R. K.; Jeganmohan, M. Chem. Commun. 2014, 50. 2442. |
| [6] | (a) Ge, J.; Wang, X.; Liu, T.; Shi, Z.; Xiao, Q.; Yin, D. RSC Adv. 2016, 6, 19571. |
| [6] | (b) Sahoo, M. K.; Midya, S. P.; Landge, V. G.; Balaraman, E. Green Chem. 2017, 19, 2111. |
| [6] | (c) Candito, D. A.; Lautens, M. Angew. Chem. Int. Ed. 2009, 48, 6713. |
| [6] | (d) Xu, Z.; Hang, Z.; Liu, Z.-Q. Org. Lett. 2016, 18, 4470. |
| [6] | (e) Li, Z.; Fan, F.; Yang, J.; Liu, Z.-Q. Org. Lett. 2014, 16, 3396. |
| [6] | (f) Xu, Z.; Yan, C.; Liu, Z.-Q. Org. Lett. 2014, 16, 5670. |
| [7] | (a) Dai, Q.; Yun, J.-T.; Feng, X.; Jiang, Y.; Yang, H.; Cheng, J. Adv. Synth. Catal. 2014, 356, 3341. |
| [7] | (b) Sha, W.; Yu, J.-T.; Jiang, Y.; Yang, H.; Cheng, J. Chem. Commun. 2014, 50, 9179. |
| [7] | (c) Tu, H.-Y.; Liu, Y.-R.; Chu, J.-J.; Hu, B.-L.; Zhang, X.-G. J. Org. Chem. 2014, 79, 9907. |
| [7] | (d) Jiang, H.; An, X.; Tong, K.; Zhang, Y.; Yu, S. Angew. Chem. Int. Ed. 2015, 54, 4055. |
| [7] | (e) Cao, J.-J.; Zhu, T.-H.; Wang, S.-Y.; Gu, Z.-Y.; Wang, X.; Ji, S.-J. Chem. Commun. 2014, 50, 6439. |
| [7] | (f) Zhang, B.; Mück-Lichtenfeld, C.; Daniliuc, C. G.; Studer, A. Angew. Chem. Int. Ed. 2013, 52, 10792. |
| [7] | (g) Sun, X.; Yu, S. Chem. Commun. 2016, 52, 10898. |
| [7] | (h) Wang, Y.-F.; Lonca, G. H.; Runigo, M. L.; Chiba, S. Org. Lett., 2014, 16, 4272. |
| [7] | (i) Lu, L.; Zhou, B.; Jin, H.; Liu, Y. Chin. J. Org. Chem. 2019, 39, 515.(in Chinese). |
| [7] | ( 陆露露, 周丙伟, 金红卫, 刘运奎, 有机化学, 2019, 39, 515.) |
| [7] | (j) Shi, D.; Bao, H.; Xu, Z.; Liu, Y. Chin. J. Org. Chem. 2017, 37, 1290(in Chinese). |
| [7] | ( 施冬冬, 鲍汉扬, 徐峥, 刘运奎, 有机化学, 2017, 37, 1290.) |
| [8] | (a) Evoniuk, C. J.; dos Passos, Gomes, Hill, G.; S, P.; Fujita, S.; Hanson, K.; Alabugin, I.V. J. Am. Chem. Soc. 2017, 139, 16210. |
| [8] | (b) Evoniuk, C. J.; Hill, S. P.; Hanson, K.; Alabugin, I.V. Chem. Commun. 2016, 52, 7138. |
| [8] | (c) Zhang, L.; Ang, G. Y.; Chiba, S. Org. Lett. 2010, 12, 3682. |
| [9] | (a) Liu, Y.-Y.; Song, R.-J.; Wu, C.-Y.; Gong, L.-B.; Hu, M.; Wang, Z.-Q.; Xie, Y.-X.; Li, J.-H. Adv. Synth. Catal. 2012 354, 347. |
| [9] | (b) Borah, A.; Gogoi, P. Eur. J. Org. Chem. 2016, 2200. |
| [9] | (c) Han, W.; Zhou, X.; Yang, S.; Xiang, G.; Cui, B.; Chen, Y. J. Org. Chem. 2015 80, 11580. |
| [9] | (d) Maestri, G.; Larraufie, M.-H.; Derat, é.; Ollivier, C.; Fensterbank, L.; Lac?te, E.; Malacria, M. Org. Lett. 2010 12, 5692. |
| [9] | (e) Portela-Cubillo, F.; Scott, J. S.; Walton, J.C. J. Org. Chem. 2008 73, 5558. |
| [9] | (f) Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Angew. Chem. Int. Ed. 2015 54, 4055. |
| [9] | (g) An, X.-D.; Yu, S. Org. Lett. 2015 17, 2692. |
| [9] | (h) Ghosh, M.; Ahmed, A.; Singha, R.; Ray, J.K. Tetrahedron Lett. 2015 56, 353. |
| [9] | (i) Portela, C. F.; Scanlan, E. M.; Scott, J. S.; Walton, J.C. Chem. Commun. 2008 4189. |
| [9] | (j) Tummatorn, J.; Krajangsri, S.; Norseeda, K.; Thongsornkleeb, C.; Ruchirawat, S. Org. Biomol. Chem. 2014 12, 5077. |
| [9] | (k) Budén, M.; Dorn, V. B.; Gamba, M.; Píerini, A. B.; Rossi, R.A. J. Org. Chem. 2010 75, 2206. |
| [9] | (l) Linsenmeier, A. M.; Williams, C. M.; Br?se, S. J. Org. Chem. 2011 76, 9127. |
| [9] | (m) McBurney, R. T.; Slawin, A. M. Z.; Smart, L. A.; Yu, Y.; Walton, J.C. Chem. Commun. 2011 47, 7974. |
| [9] | (n) Hofstra, J. L.; Grassbaugh, B. R.; Tran, Q. M.; Armada, N. R.; de Lijser, H.J. P. J. Org. Chem. 2015 80, 256. |
| [9] | (o) Chen, W.-L.; Chen, C.-Y.; Chen, Y.-F.; Hsieh, J.-C. Org. Lett. 2015 17, 1613. |
| [10] | Selected reviews: |
| [10] | (a) Yin, G.; Mu, X.; Liu, G. Acc. Chem. Res. 2016, 49, 2413. |
| [10] | (b) Egami, H.; Sodeoka, M. Angew. Chem. Int. Ed. 2014, 53, 8294. |
| [10] | (c) Shimizu, Y.; Kanai, M. Tetrahedron Lett. 2014, 55, 3727. |
| [10] | (d) McDonald, R.; Liu, G.; Stahl, S.S. Chem. Rev. 2011, 111, 2981. |
| [10] | (e) Zeni, G.; Larock, R.C. Chem. Rev. 2006, 106, 4644. |
| [10] | (f) Vlaar, T.; Ruijter, E.; Orru, R. Adv. Synth. Catal. 2011, 353, 809. |
| [10] | (g) Giri, R.; Shekhar, K.C. J. Org. Chem. 2018, 83, 3013. |
| [10] | (h) Chemler, S. R.; Bovino, M.T. ACS Catal. 2013, 3, 1076. |
| [11] | Addition of XCuOH to carbon-carbon multiple bonds, see: |
| [11] | (a) Zhang, W.; Zhang, J.; Liu, Y.; Xu, Z. Synlett. 2013, 24, 2709. |
| [11] | (b) Zhang, J.; Wu, D.; Chen, X.; Liu, Y.; Xu, Z. J. Org. Chem. 2014, 79, 4799. |
| [11] | (c) Zhang, J.; Wang, H.; Ren, S.; Zhang, W.; Liu, Y. Org. Lett. 2015, 17, 2920. |
| [11] | (d) Zhang, J.; Zhang, H.; Shi, D.; Jin, H.; Liu, Y. Eur. J. Org. Chem. 2016, 5545. |
| [11] | (e) Bao, H.; Xu, Z.; Wu, D.; Zhang, H.; Jin, H.; Liu, Y. J. Org. Chem. 2017, 82, 109. |
| [12] | Addition of XCuOH to C=O bonds, see: (f) Zhang, J.; Shi, D.; Zhang, H.; Xu, Z.; Bao, H.; Jin, H.; Liu, Y. Tetrahedron 2017, 73, 154. |
| [13] | (a) Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2015, 80, 3243. |
| [13] | (b) Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892. |
| [13] | (c) Zhou, W.; Liu, Y.; Yang, Y.; Deng, G.-J. Chem. Commun. 2012, 48, 10678. |
| [14] | (a) Srimani, D.; Leitus, G.; Ben-David, Y.; Milstein, D. Angew. Chem. Int. Ed. 2014, 53, 11092. and references cited therein. |
| [14] | (b) Julia, M.; Paris, J.-M. Tetrahedron Lett. 1973, 14, 4833. |
| [14] | (c) Blakemore, P.R. J. Chem. Soc. Perkin Trans. 2002, 2563. |
| [14] | (d) Chatterjee, B.; Bera, S.; Mondal, D. Tetrahedron: Asymmetry. 2014, 25, 1. |
| [15] | Yamanaka, M.; Nishida, A.; Nakagawa, M. J. Org. Chem. 2003, 68, 3112. |
| [16] | Qiu, J.; Wang, L.; Liu, M.; Shen, Q.; Tang, J. Tetrahedron Lett. 2011, 52, 6489. |
| [17] | Siddiqui, M. A.; Snieckus, V . Tetrahedron Lett. 1988,29, 5463. |
| [18] | Keene, B. R. T. J . Chem. Soc. 1965,3032. |
| [19] | Badger, G. M.; Sasse, W. F. H. J . Chem. Soc. 1957,4. |
| [20] | Maestri, G.; Larraufie, M.-H.; Derat, E.; Ollivier, C.; Fensterbank, L.; Lacote, E.; Malacria, M. Lett. Org. . 2010, 12, 5692. |
| [21] | Coombs, M. M. J . Chem. Soc. 1958,3454. |
| [22] | Arcus, C. L.; Coombs, M. M.; Evans, J. V. J . Chem. Soc. 1956,1498. |
| [23] | Kessar, S. V.; Grupta, Y. P.; Balakrishnan, P.; Sawal, K. K.; Mohammad, T Dutt, M..; J. Org. Chem. 1988, 53, 1708. |
/
| 〈 |
|
〉 |