

无金属催化快速合成2-芳酰氨基萘并[1, 2-d]噻唑
收稿日期: 2019-04-11
网络出版日期: 2019-07-03
基金资助
国家自然科学基金(21271035);安徽省自然科学基金(1808085QB48);安徽省有机化学省级教学团队(2017jxtd051)
Metal-Free Rapid Synthesis of 2-Aroylamino Naphtho[1, 2-d]thiazoles
Received date: 2019-04-11
Online published: 2019-07-03
Supported by
the National Natural Science Foundation of China(21271035);the Natural Science Foundation of Anhui Province(1808085QB48);the Provincial Teaching Team of Organic Chemistry(2017jxtd051)
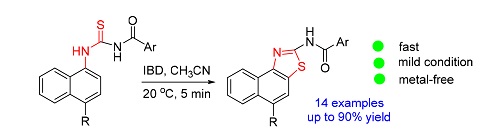
报道了在室温条件下,二醋酸碘苯促进3-[1-(4-取代萘基)]-1-芳酰基硫脲自身关环得到一系列2-芳酰氨基萘并[1,2-d]噻唑衍生物.该方法具有反应条件温和,反应迅速,操作简单,原子利用率高,无需金属催化以及底物范围广等优点.该方法也为萘并[1,2-d]噻唑衍生物的合成提供了新的高效途径.
关键词: 萘并[1, 2-d]噻唑; 硫脲; 二醋酸碘苯; 无金属催化
刘天宝 , 彭艳芬 , 桂美芳 , 章敏 . 无金属催化快速合成2-芳酰氨基萘并[1, 2-d]噻唑[J]. 有机化学, 2019 , 39(11) : 3199 -3206 . DOI: 10.6023/cjoc201904029
A novel and efficient approach has been developed to synthesize 2-aroylamino naphtho[1, 2-d]thiazole compounds through the reaction between 3-[1-(4-substituted naphthyl)]-1-aroylthiourea and iodosobenzene diacetate (IBD) under ambient air. A library of naphtho[1, 2-d]thiazole derivatives having a variety of substituents has been synthesized. A plausible reaction pathway has been predicted. This reaction offers a metal-free synthesis, broad substrate scope, easily accessible reactants, excellent regioselectivity, room temperature reaction conditions under ambient air. The reported method is the efficient approach for the synthesis of naphtho[1, 2-d]thiazole derivatives.

Key words: naphtho[1, 2-d]thiazole; thiourea; iodosobenzene diacetate; metal-free
| [1] | (a) Joy, H. B.; Bogert, M. T. J. Org. Chem. 1936, 1, 236. |
| [1] | (b) Keyes, G. H.; Brooker, L. G. S. J. Am. Chem. Soc. 1937, 59, 74. |
| [1] | (c) Akins, D. L.; ?z?elik, S.; Zhu, H.-R.; Guo, C. J. Phys. Chem. A 1997, 101, 3251. |
| [1] | (d) Urano, T.; Hino, E. Imaging Sci. J. 1999, 47, 127. |
| [1] | (e) Huang, W.; Zhang, X.-H.; Wang, L.-Y.; Zhai, G.-H.; Wen, Z.-Y.; Zhang, Z.-X. J. J. Mol. Struct. 2010, 977, 39. |
| [2] | (a) Perrone, R.; Berardi, F.; Colabufo, N. A.; Tortorella, V.; Fornaretto, M. G.; Caccia, C.; Mcarthur, R. A. Eur. J. Med. Chem. 1997, 32, 739. |
| [2] | (b) Li, Z.-G.; Yang, Q.; Qian, X.-H. Bioorg. Med. Chem. 2005, 13, 3149. |
| [2] | (c) Hu, H.; Owwns, E. A.; Su, H.-R.; Yan, L.-L.; Lecitz, A.; Zhao, X.-Y.; Henary, M.; Zheng, Y. J. G. J. Med. Chem. 2015, 58, 1228. |
| [3] | (a) El-Shishtawy, R. M.; Asiri, A. M.; Basaif, S. A.; Sobahi, T. R. Spectrochim. Acta A 2010, 75, 1605. |
| [3] | (b) Aiken, S.; Allsopp, B.; Booth, K.; Gabbutt, C. D.; Heron, B. M.; Rice, C. R. Tetrahedron 2014, 70, 9352. |
| [3] | (c) Yang, W.; Liu, C.-L.; Gao, Q.-Y.; Du, J.-Y.; Shen, P.; Liu, Y.; Yang, C.-Y. Opt. Mater. 2017, 66, 623. |
| [3] | (d) Liu, C.-L.; Yang, W.; Gao, Q.-Y.; Du, J.-Y.; Luo, H.-J.; Liu, Y.; Yang, C.-Y. J. Lumin. 2018, 197, 193. |
| [4] | (a) Lau, P. T. S.; Gompf, T. E. J. Org. Chem. 1970, 35, 4103. |
| [4] | (b) Ulrich, P.; Cerami, A. J. Med. Chem. 1982, 25, 654. |
| [4] | (c) El-Taweel, F. M. A. A.; Elnagdi, M. H. J. Heterocycl. Chem. 2001, 38, 981. |
| [4] | (d) Al-Saleh, B.; El-Apasery, M. A.; Abdel-Aziz, R. S.; Elnagdi, M. H. J. Heterocycl. Chem. 2005, 42, 563. |
| [5] | Zhang L.-F.; Ni Z.-H.; Li D.-Y.; Qin Z.-H.; Wei X.-Y. Chin. Chem. Lett. 2012, 23 281. |
| [6] | Jonaghani M. Z.; Boeini H. Z. Spectrochim. Acta A 2017, 178 66. |
| [7] | (a) Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328. |
| [7] | (b) Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299. |
| [8] | (a) Cho, S. H.; Yoon, J.; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996. |
| [8] | (b) Farid, U.; Wirth, T. Angew. Chem., Int. Ed. 2012, 51, 3462. |
| [8] | (c) Saito, E.; Matsumoto, Y.; Nakamura, A.; Namera, Y.; Nakada, M. Org. Lett. 2018, 20, 692. |
| [8] | (d) Kitamura, T.; Miyake, A.; Muta, K.; Oyamada, J. J. Org. Chem. 2017, 82, 11721. |
| [8] | (e) Liu, T.-B.; Peng, Y.-F.; Wang, Y.-J.; Yong, J.-Y.; Wang, X. Chin. J. Org. Chem. 2018, 38, 969 (in Chinese). |
| [8] | (刘天宝, 彭艳芬, 王雅洁, 雍家远, 汪新, 有机化学, 2018, 38, 969.) |
| [9] | (a) Alvarado, J.; Fournier, J.; Zakarian, A. Angew. Chem., Int. Ed. 2016, 55, 11625. |
| [9] | (b) Zhang, H.; Shen, J.; Cheng, G.; Feng, Y.; Cui, X. Org. Lett. 2018, 20, 664. |
| [9] | (c) Zhang, X.; Hou, W.; Zhang-Negrerie, D.; Zhao, K.; Du, Y. Org. Lett. 2015, 17, 5252. |
| [9] | (d) Zheng, Y.; Li, X.; Ren, C.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. J. Org. Chem. 2012, 77, 10353. |
| [9] | (e) Zhang, N.; Cheng, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. J. Org. Chem. 2014, 79, 10581. |
| [10] | (a) Mariappan, A.; Rajaguru, K.; Roja, S. S.; Muthusubramanian, S.; Bhuvanesh, N. Eur. J. Org. Chem. 2016, 81, 302. |
| [10] | (b) Downer-Riley, N. K.; Jackson, Y. A. Tetrahedron 2008, 64, 7741. |
| [10] | (c) Guo, W.-S.; Gong, H.; Zhang, Y.-A.; Wen, L.-R.; Li, M. Org. Lett. 2018, 20, 6394. |
| [10] | (d) Chinchilla, R.; Nájera, C.; Yus, M. Chem. Rev. 2004, 104, 2667. |
| [10] | (e) Xie, H.; Cai, J.-H.; Wang, Z.-L.; Huang, H.-W.; Deng, G.-J. Org. Lett. 2016, 18, 2196. |
| [10] | (f) Wipf, P.; Venkatraman, S. J. Org. Chem. 1996, 61, 8004. |
| [10] | (g) Kumar, D.; Kumar, N. M.; Chang, K.-H.; Gupta, R.; Shah, K. Bioorg. Med. Chem. Lett. 2011, 21, 5897. |
| [10] | (h) Bose, D. S.; Idrees, M. J. Org. Chem. 2006, 71, 8261. |
| [11] | Kumar D.; Kumar N. M.; Chang K.-H.; Gupta R.; Shah K. Bioorg. Med. Chem. Lett. 2011, 21 5897. |
| [12] | Liu T.-B.; Peng Y.-F. Chin. J. Chem. Educ. 2018, 39 36. |
| [12] | 刘 天宝; 彭 艳芬 化学教育 2018, 39 36. |
/
| 〈 |
|
〉 |