

烯基膦氧配位的第10族过渡金属配位化合物的合成和性质
收稿日期: 2019-05-01
修回日期: 2019-06-19
网络出版日期: 2019-07-09
基金资助
国家自然科学基金(No.21871070)和海南省自然科学基金(No.219MS005)资助项目.
Synthesis and Reactivity of Group 10 Transition Metal Complexes with Alkenylphosphoryl Compounds
Received date: 2019-05-01
Revised date: 2019-06-19
Online published: 2019-07-09
Supported by
Project supported by the National Natural Science Foundation of China (No. 21871070), and the Natural Science Foundation of Hainan Province (No. 219MS005).
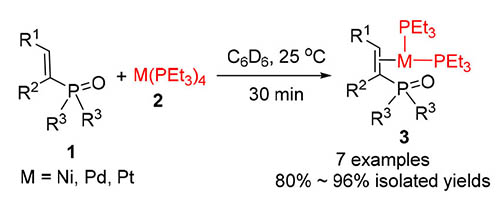
在室温下,烯基膦氧化合物能够快速地替换掉零价钯配合物Pd(PEt3)4中的两个膦配体,高产率地合成对应的烯基膦氧配位的钯配位化合物3a~3e.烯基膦氧配位的镍和铂配合物3f和3g也能够通过类似的反应方法获得.3e能够与HOAc反应缓慢地释放出烯基膦氧化合物;在相似的条件下再加入2倍量的PEt3,反应能够进行彻底,并缓慢沉淀出一个季磷盐4.与Pd(PEt3)4类似,3d也能够与苯乙炔和亚磷酸二乙酯发生钯氢化反应生成对应的乙烯基-Pd-磷酰基配位化合物,但是反应速度相对比较慢,该结果说明,在钯催化炔烃膦酰化反应中,生成的烯基膦氧化合物将一定程度上抑制催化循环中钯氢化环节的发生.所合成的配位化合和季磷盐4都经过核磁表征,其中两个化合物的分子结构还经过X射线衍射技术确认.
陈铁桥 , 刘龙 , 黄添增 , 韩立彪 . 烯基膦氧配位的第10族过渡金属配位化合物的合成和性质[J]. 有机化学, 2019 , 39(8) : 2183 -2187 . DOI: 10.6023/cjoc201905001
Alkenylphosphoryl compounds could quickly replace two phosphine ligands in Pd(PEt3)4 at room temperature to produce the corresponding palladium complexes 3a~3e in high yields. Similar nickel and platinium complexes 3f and 3g were also synthesized by the reaction. Complex 3e could react with HOAc to release alkenylphosphoryl compounds gradually. By further addition of 2 equiv. of PEt3, the reaction could take place completely, generating a phosphoniun 4 as a precipitation. Being similar to Pd(PEt3)4, 3d could also undergo hydropalladation with the combination of phenylacetylene and (EtO)2P(O)H, forming the expected vinyl-Pd-phosphoryl complex, but with a slower reaction rate. The result indicated that alkenylphosphoryl compounds generated from the palladium-catalyzed hydrophosphorylation of alkynes with H-phosphonates might play a negative role in the hydropalladation step of the catalytic cycle. Complexes 3a~3g and phosphonium 4 were charaterized by NMR analysis. The structures of 3a and 4 were also confirmed by X-ray technology.

[1] For selected book and reviews, see:(a) Lawrance, G. A. Introduction to Coordination Chemistry, Wiley, Chichester, UK, 2010.
(b) Burgess, J.; Steel, P. J. Coord. Chem. Rev. 2011, 255, 2094.
(c) Steobenau, E. J.; Jordan, R. F. J. Am. Chem. Soc. 2006, 128, 8162.
(d) Harvey, B. G.; Ernst, R. D. Eur. J. Inorg. Chem. 2017, 2017, 1205.
(e) Defieber, C.; Grutzmacher, H.; Carreira, E. M. Angew. Chem., Int. Ed. 2008, 47, 4482.
(f) Johnson, J. B.; Rovis, T. Angew. Chem., Int. Ed. 2008, 47, 840.
(g) Feng, X.; Du, H. Chin. J. Org. Chem. 2015, 35, 259(in Chinese). (冯向青,杜海峰,有机化学, 2015, 35, 259.)
[2] For selected reviews, see:(a) Dembitsky, V. M.; Quntar, A. A. A. A.; Haj-Yehia, A.; Srebnik, M. Mini-Rev. Org. Chem. 2005, 2, 91.
(b) Minami, T.; Motoyoshiya, J. Synthesis 1992, 333.
(c) Wang, H.-Q.; Liu, Z.-J. Chin. J. Org. Chem. 2003, 23, 321(in Chinese). (王宏青,刘钊杰,有机化学, 2003, 23, 321.)
[3] (a) Al-Quntar, A. A.-A.; Baum, O.; Reich, R.; Srebnik, M. Arch. Pharm. 2004, 337, 76.
(b) Liu, Z.; MacRitchie, N.; Pyne, S.; Pyne, N. J.; Bittman, R. Bioorg. Med. Chem. 2013, 21, 2503.
(c) Harnden, M. R.; Parkin, A.; Parratt, M. J.; Perkins, R. M. J. Med. Chem. 1993, 36, 1343.
(d) Tonelli, F.; Lim, K. G.; Loveridge, C.; Long, J.; Pitson, S. M.; Tigyi, G.; Bittman, R.; Pyne, S.; Pyne, N. J. Cell. Signalling 2010, 22, 1536.
[4] (a) Kosolapoff, G. M. J. Am. Chem. Soc. 1948, 70, 1971.
(b) Mulla, K.; Aleshire, K. L.; Forster, P. M.; Kang, J. Y. J. Org. Chem. 2016, 81, 77 and references cited therein.
[5] Han, L.-B.; Tanaka, M. J. Am. Chem. Soc. 1996, 118, 1571.
[6] (a) Coudray, L.; Montchamp, J.-L. Eur. J. Org. Chem. 2008, 3601.
(b) Delacroix, O.; Gaumont, A. C. Curr. Org. Chem. 2005, 9, 1851.
(c) Xu, Q.; Han, L.-B. J. Organomet. Chem. 2011, 696, 130.
[7] http://www.katayamakagaku.co.jp/products/chemicalproducts/flameretardant/index.html
[8] Chen, T.; Zhao, C.-Q.; Han, L.-B. J. Am. Chem. Soc. 2018, 140, 3139.
[9] (a) Stewart, I. C.; Bergman, R. G.; Toste, F. D. J. Am. Chem. Soc. 2003, 125, 8696.
(b) Okuma, K.; Izaki, T. Bull. Chem. Soc. Jpn. 2005, 78, 1831.
(c) Kentaro, O., Koichiro, Y.; Toshiharu, I.; Keiko, Y.; Kosei, S. Bull. Chem. Soc. Jpn. 2007, 80, 1785.
(d) Arisawa, M.; Yamaguchi, M. J. Am. Chem. Soc. 2006, 128, 50.
(e) Arisawa, M.; Yamaguchi, M. In ACS Symposium Series, Ed.:Laali, K. K., American Chemical Society, Washington, DC, 2007, Vol. 965, Chapter 22, pp. 477~492.
[10] For a review, see:Guo, H.; Fan, Y. C.; Sun, Z.; Wu, Y.; Kwon, O. Chem. Rev. 2018, 118, 10049.
[11] Both electronic and steric factors affect the regioselectivity of hydropalladation, for details, please see Ref.
[8] . A related work on the basis of DFT calculation was also reported, see:Zhang, H.; Bao, X. RSC Adv. 2015, 5, 84636.
/
| 〈 |
|
〉 |