

立体位阻效应导致的苯酚的区域选择性溴化
收稿日期: 2019-07-26
修回日期: 2019-08-22
网络出版日期: 2019-09-05
基金资助
河南省科技攻关(192102310031);河南省高等学校重点科研(19B150018);信阳师范学院“南湖学者奖励计划”青年项目;信阳师范学院青年骨干教师计划资助项目(2018GGJS-05)
Steric Hindrance Effect Leading to Regioselective Bromination of Phenols with HBr
Received date: 2019-07-26
Revised date: 2019-08-22
Online published: 2019-09-05
Supported by
Project supported by the Key Scientific and Technological Project of Henan Province(192102310031);The Scientific Research Project of Henan Province(19B150018);The Nanhu Scholars Program for Young Scholars of Xinyang Normal University;The Young Core Instructor Program of Xinyang Normal University(2018GGJS-05)
马献涛 , 周坤洁 , 任梦娟 , 王梦雨 , 于静 . 立体位阻效应导致的苯酚的区域选择性溴化[J]. 有机化学, 2019 , 39(10) : 2796 -2801 . DOI: 10.6023/cjoc201907038
A mild and regioselective bromination of phenols with the cheap and easily-available HBr is developed. By replacing the common used dimethyl sulfoxide (DMSO) with sulfoxides bearing sterically hindered substituents, the desired brominated phenols could be obtained in moderate to high yields with up to 99/1 regioselectivity. This method could be easily scaled up to 50 mmol scale and has the potential to isolate the desired product by simple extraction and recrystallization, showing great practicality of this new method.
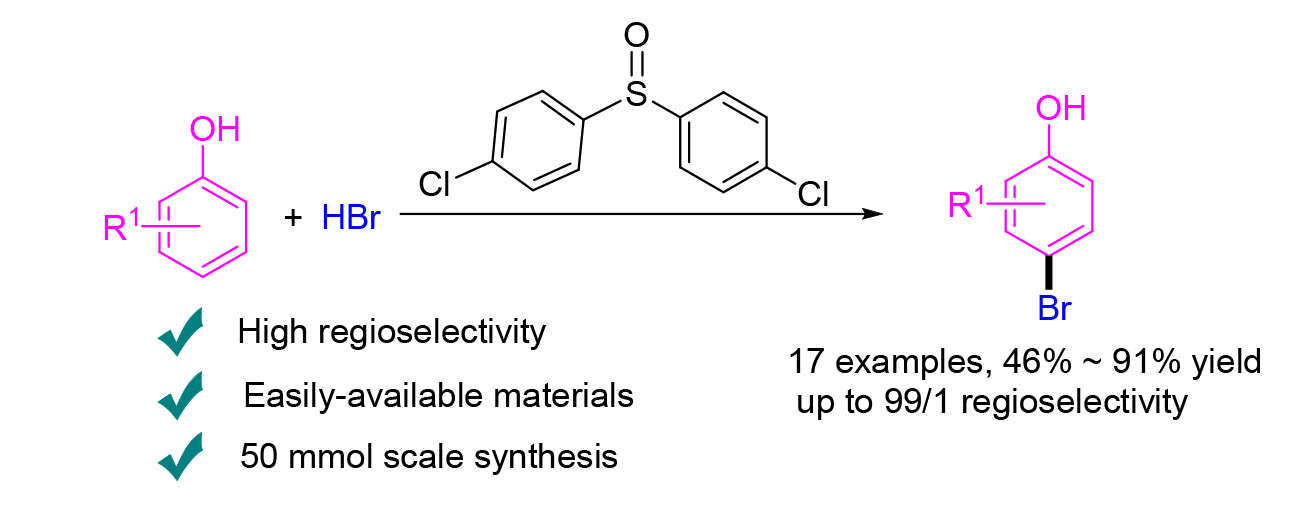
Key words: phenol; regioselective bromation; HBr; sulfoxide; steric hindrance effect
| [1] | Fusetani, N.; Matsunaga, S. Chem. Rev. 1993, 93, 1793. |
| [1] | (b) Segraves, E. N.; Shah, R. R.; Segraves, N. L.; Johnson, T. A.; Whitman, S.; Sui, J. K.; Kenyon, V. A.; Cichewicz, R. H.; Crews, P.; Holman, T.R. J. Med. Chem. 2004, 47, 4060. |
| [1] | (c) Akai, S.; Kakiguchi, K.; Nakamura, Y.; Kuriwaki, I.; Dohi, T.; Harada, S.; Kubo, O.; Morita, N.; Kita, Y. Angew. Chem., Int. Ed. 2007, 46, 7458. |
| [1] | (d) Qian, S.; Ma, Y.; Gao, S.; Luo, J. Chin. J. Org. Chem. 2018, 38, 1930.(in Chinese). |
| [1] | ( 钱少平, 马尧睿, 高姗姗, 骆钧飞, 有机化学, 2018, 38, 1930.) |
| [1] | (e) Zhou, P.; Hou, A.; Wang, Y. Chin. J. Org. Chem. 2018, 38, 156(in Chinese). |
| [1] | ( 周鹏飞, 侯爱君, 王洋, 有机化学, 2018, 38, 156.) |
| [2] | For reviews:see: (a) Smith, K.; El-HitiI, G.A. Curr. Org. Synth. 2004, 1, 253. |
| [2] | (b) Saikia, A. J.; Borah, P.P. Chem. Rev. 2016, 116, 6837. |
| [3] | For a review see:(a) Luo, J.; Xu, X.; Zhao, Y.; Liang, H. Chin. J. Org. Chem. 2017, 37, 2873 (in Chinese). |
| [3] | ( 骆钧飞, 徐星, 赵延超, 梁洪泽, 有机化学, 2017, 37, 2873 ) |
| [3] | For selected recent reports, see: (b) Okada, Y.; Yokozawa, M.; Akiba, M.; Oishi, K.; O-kawa, K.; Akeboshi, T.; Kawamura, Y.; Inokuma, S.; Nakamura, Y.; Nishimura, J. Org. Biomol. Chem. 2003, 1, 2506. |
| [3] | (c) Bovonsombat, P.; Ali, R.; Khan, C.; Leykajarakul, J.; Pla-on, K.; Aphimanchindakul, S.; Pungcharoenpong, N.; Timsuea, N.; Arunrat, A.; Punpongjareorn, N. Tetrahedron. 2010, 66, 6928. |
| [3] | (d) Racys, D. T.; Warrilow, C. E.; Pimlott, S. L.; Sutherland, A. Org. Lett. 2015, 17, 4782. |
| [3] | (e) Nishimura, J.; Tang, R.-J.; Milcent, T.; Crousse, B. J. Org. Chem. 2018, 83, 930. |
| [4] | For a review, see: Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; Garneau-Tsodikova, S.; Walsh, C.T. Chem. Rev. 2006, 106, 3364. |
| [5] | For reviews, see: (a) Podgors?ek, A.; Zupan, M.; Iskra, J. Angew. Chem., Int. Ed. 2009, 48, 8424. |
| [5] | (b) Zhang, G.; Wang, Y.; Ding, C.; Liu, R.; Liang, X. Chin. J. Org. Chem. 2011, 31, 804 (in Chinese). |
| [5] | For selected recent reports, see: (c) Werf, A.; Selander, N. Org. Lett. 2015, 17, 6210. |
| [5] | For selected recent reports, see: (c) Werf, A.; Selander, N. Org. Lett. 2015, 17, 6210. |
| [5] | (d) Satkar, Y.; Ramadoss, V.; Nahide, P. D.; García-Medina, E.; Juárez-Ornelas, K. A.; Alonso-Castro, A. J.; Chávez-Rivera, R.; Jiménez-Halla, J. O. C.; Solorio-Alvarado, C.R. RSC Adv. 2018, 8, 17806. |
| [5] | (e) Sorabad, G. S.; Maddani, M.R. New J. Chem. 2019, 43, 6563. |
| [5] | (f) Walter, C.; Fallows, N.; Kesharwani, T. ACS Omega, 2019, 4, 6538. |
| [5] | (g) Semwal, R.; Ravi, C.; Kumar, R.; Meena, R.; Adimurthy, S. J. Org. Chem. 2019, 84, 792. |
| [5] | (h) Satkar, Y.; Yera-Ledesma, L. F.; Mali, N.; Patil, D.; Navarro-Santos, P.; Segura-Quezada, L. A.; Ramírez-Morales, P. I.; Solorio-Alvarado, C.R. J. Org. Chem. 2019, 84, 4149. |
| [5] | (i) Segura-Quezada, A.; Satkar, Y.; Patil, D.; Mali, N.; Wrobel, K.; González, G.; Zárraga, R.; Ortiz-Alvarado, R.; Solorio-Alvarado, C.R. Tetrahedron Lett. 2019, 60, 1551. |
| [6] | For selected recent reports, see: (a) Mal, K.; Sharma, A.; Maulik, P. R.; Das, I. . Chem.-Eur. J 2013, 20, 662. |
| [6] | (b) Liu, C.; Dai, R.; Yao, G.; Deng, Y.J. Chem. Res. 2014, 38, 593. |
| [6] | (c) Song, S.; Li, X.; Sun, X.; Yuan, Y.; Jiao, N. Green Chem. 2015, 17, 3285. |
| [6] | (d) Karki, M.; Magolan, J. J. Org. Chem. 2015, 80, 3701. |
| [6] | (e) Mal, K.; Kaur, A.; Haque, F.; Das, I. J. Org. Chem. 2015, 80, 640. |
| [6] | (f) Sorabad, G. S.; Maddani, M.R. New J. Chem. 2019, 43, 6563. |
| [7] | (a) Pandit, P. K.; Gayen, S.; Khamarui, S.; Chatterjee, N.; Maiti, D.K. Chem. Commun. 2011, 47, 6933. |
| [7] | (b) Iskra, J.; Murphree, S.S. Tetrahedron Lett. 2017, 58, 645. |
| [7] | (c) Xin, H.; Yang, S.; An, B.; An, Z. RSC Adv. 2017, 7, 13467. |
| [7] | (d) Tomizuka, A.; Moriyama, K. Adv. Synth. Catal. 2019, 361, 1447. |
| [7] | (e) Xin, H.; Hu, L.; Yu, J.; Sun, W.; An, Z. Catal. Commun. 2017, 93, 1. |
| [7] | (f) Kajita, H.; Togni, A. ChemistrySelect. 2017, 2, 1117. |
| [7] | (g) Cao, L.; Liu, B.; Liu, W.; Yao, G.; Cheng, Q. Chin. J. Org. Chem. 2011, 31, 2178.(in Chinese). |
| [7] | ( 曹志凌, 刘冰, 刘玮炜, 姚国伟, 程青芳, 有机化学, 2011, 31, 2178.) |
| [8] | Song, S.; Sun, X.; Li, X.; Yuan, Y.; Jiao, N. Org. . Lett 2015, 17, 2886. |
| [9] | For reviews, see: (a) Huang, Z.; Lumb, J.-P. ACS Catal. 2019, 9, 521. |
| [9] | (b) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2, 1107. |
| [9] | (c) Yanagi, T.; Nogi, K.; Yorimitsu, H. Tetrahedron Lett. 2018, 59, 2951. |
| [10] | (a) Ma, X.-T.; Tian, S.-K. Adv. Synth. Catal. 2013, 355, 337. |
| [10] | (b) Ma, X.; Yu, J.; Jiang, M.; Wang, M.; Tang, L.; Wei, M.; Zhou, Q. Eur. J. Org. Chem. 2019,4593. |
| [11] | Chauhan and coworkers reported a regioselective bromination of phenol with HBr at room temperature. The target 4-bromophenol could be obtained in 89% yield, but no experimental details could be found in the literature, see: Srivastava, S. K.; Chauhan P. M. S.; Bhaduri, A. P. Chem. Commun.1996, 2679 for details. We attempted for some times, but the target 3a was obtained only in low yield by using DMSO as a solvent at room temperature. |
| [12] | Our experimental results are consistent with Jiao’s observation, ie the use of stoichiometric DMSO as the oxidant instead of as the solvent can greatly improve the reaction efficiency and selectivity, see Ref. [8]. |
| [13] | Kakarla, R.; Dulina, R. G.; Hatzenbuhler, N. T.; Hui, Y. W.; Sofia, M.J. J. Org. Chem. 1996, 61, 8347. |
| [14] | Choudhury, L. H.; Parvin, T.; Khan, A. T. Tetrahedron 2009, 65, 9513. |
| [15] | Ghiaci, M.; Sedaghat, M. E.; Ranjbari, S.; Gil, A. Appl. Catal. A: Gen. 2010, 384, 18. |
| [16] | Mabic, S.; Lepoittevin, J.-P. Tetrahedron Lett. 1995, 36, 1705. |
| [17] | Lou, S.-J.; Chen, Q.; Wang, Y.-F.; Xu, D.-Q.; Du, X.-H.; He, J.-Q.; Mao, Y.-J.; Xu, Z.-Y. ACS Catal. 2015, 5, 2846. |
| [18] | Xiong, X.; Yeung, Y.-Y. ACS Catal. 2018, 8, 4033. |
| [19] | Carrigan, M. D.; Sarapa, D.; Smith, R. C.; Wieland, L. C.; Mohan, R.S. J. Org. Chem. 2002, 67, 1027. |
| [20] | Yang, Y.; Lin, Y.; Rao, Y. Org. Lett. 2012, 14, 2874. |
| [21] | Diemer, V.; Begaud, M.; Leroux, F. R.; Colobert, F . Eur. J. Org. Chem. 2011,341. |
| [22] | Kajita, H.; Togni, A. ChemistrySelect 2017, 2, 1117. |
| [23] | Kerr, D. J.; Willis, A. C.; Flynn, B.L. Org. Lett. 2004, 6, 457. |
| [24] | Liu, Y.; Kim, J.; Seo, H.; Park, S.; Chae, J. Adv. Synth. Catal. 2015, 357, 2205. |
/
| 〈 |
|
〉 |