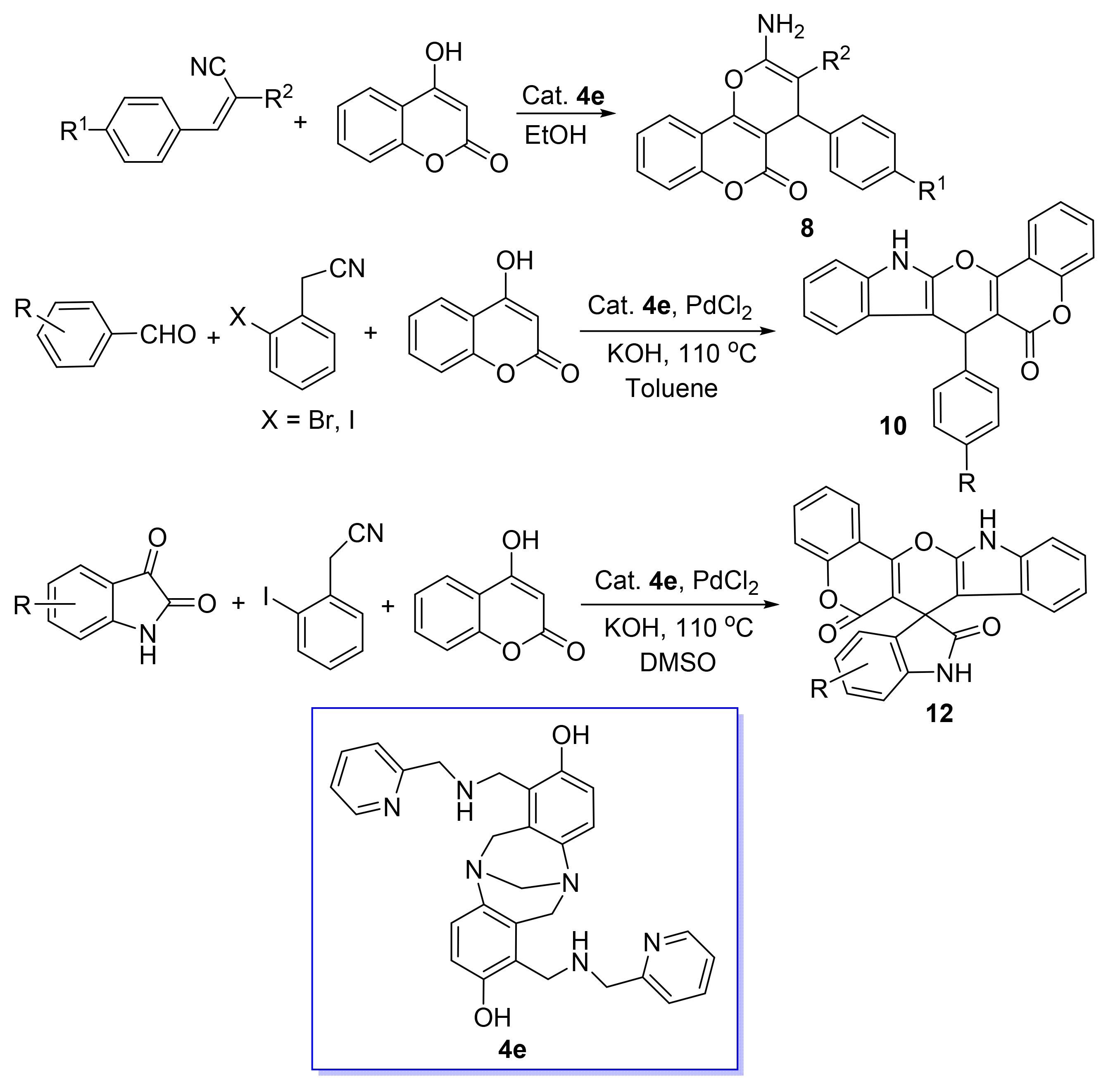
1,7-Bis(N-substituted-aminomethyl)-2,8-dihydroxy-Tröger's bases (4) were synthesized and used as efficient organocatalyst for the Aldol reaction of 4-hydroxylcoumarin and 2-benzylidenemalononitrile (or methyl(ethyl)-2-cyano-3-phenylacrylate) to afford 2-amino-4-aryl-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitriles (carboxylates) (8). Subse-quently, they were used as the efficient ligand to promote the Pd-catalyzed Aldol-Ullmann reaction to give 7-aryl-7,12-dihydro-6H-chromeno[3',4':5,6]pyrano[2,3-b]indol-6-one (10) and 5'(or 5',7')-substituted-6H,12H-spiro[chromeno[3',4':5,6]-pyrano[2,3-b]indole-7,3'-indoline]-2',6-dione (12), respectively. The anti-cancer activity on human three positive breast cancer cells (MCF-7), human three negative breast cancer cells (MDA-MB-231), human hepatoma cells (HepG2), human hepatoma cells (MHCC-97H) and cytotoxicity on human hepatocyte cells (LO2) of catalyst 4 and all products in vitro were evaluated. 1,7-Bis((methylamino)methyl)-6H,12H-5,11-methanodibenzo[b,f] [1,5]diazocine-2,8-diol (4b) had selective inhibition (inhi-bition rate>30%) on MCF-7 cells while 1,7-bis(((1-phenylethyl)amino)methyl)-6H,12H-5,11-methanodibenzo[b,f] [1,5]diazo-cine-2,8-diol (4d) and 1,7-bis(((pyridin-2-ylmethyl)amino)methyl)-6H,12H-5,11-methanodibenzo[b,f] [1,5]diazocine-2,8-diol (4e) had selective inhibition on MDA-MB-231 cells. 2-Amino-5-oxo-4-(3,4,5-trimethoxyphenyl)-4H,5H-dihydropyrano[3,2-c]chromene-3-carbonitrile (8q) had strong inhibitory effects on three kinds of cancer cells except MDA-MB-231 while 2-amino-4-(4-bromophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitrile (8a), 2-amino-4-(2,4-dichlorophenyl)-5-oxo-4H,5H-dihydropyrano[3,2-c]chromene-3-carbonitrile (8e), 2-amino-4-(3-fluorophenyl)-5-oxo-4H,5H-pyrano[3,2-c]chrome-ne-3-carbonitrile (8m) and 2-amino-4-(3-bromophenyl)-5-oxo-4H,5H-dihydropyrano[3,2-c]chromene-3-carbonitrile (8n) had strong inhibitory effects on four kinds of cancer cells. However, all the compounds showed cytotoxicity to normal LO2 cells which prompts the necessary of structure modification to reduce the toxicity.
[1] Hassanpour, A.; Hosseinzadeh-Khanmiri, R.; Ghorbanpour, K.; Abolhasani, J.; Oskoei, Y. M. Iran. J. Chem. Chem. Eng. 2016, 35, 39.
[2] Manvar, A.; Bavishi, V.; Radadiya, A.; Patel, J.; Vora, V.; Dodia, N.; Rawal, K.; Shah, A. Bioorg. Med. Chem. 2011, 16, 4728.
[3] Vekariya, H. R.; Patel, D. H. Synth. Commun. 2014, 44, 2756.
[4] Sashidhara, K. V.; Kumar, A.; Chatterjee, M.; Rao, K. B.; Singh, S.; Verma, A. K.; Palit, G. Bioorg. Med. Chem. 2011, 7, 1937.
[5] Zheng, Y.; Qiu, L.; Hong, K.; Dong, S.; Xu, X. Chem. Eur. J. 2018, 24, 6705.
[6] Zaki, R. M.; Elossaily, Y. A.; Kamal El-Dean, A. M. Russ. J. Bioorg. Chem. 2012, 38, 639.
[7] Zolfigol, M. A.; Safaiee, M.; Bahraminejad, N. J. New J. Chem. 2016, 40, 5071.
[8] Brahmachari, G.; Banerjee, B. ACS Sustain. Chem. Eng. 2013, 2, 411.
[9] Keri, R. S.; Sasidhar, B. S.; Nagaraja, B.; Santos, M. M. Eur. J. Org. Chem. 2015, 100, 257.
[10] Chen, C.; Lu, M.; Liu, Z.; Wan, J.; Tu, Z.; Zhang, T.; Yan, M. Open J. Med. Chem. 2013, 3, 128.
[11] Nasr, T.; Bondock, S.; Youns, M. Eur. J. Med. Chem. 2014, 76, 539.
[12] Wickel, S. M.; Citron, C. A.; Dickschat, J. S. Eur. J. Org. Chem. 2013, 14, 2906.
[13] Kumar, D.; Reddy, V. B.; Sharad, S.; Dube, U.; Kapur, S. A. Eur. J. Med. Chem. 2009, 44, 3805.
[14] Khaleghi-Abbasabadi, M.; Azarifar, D. Res. Chem. Intermed. 2019, 45, 2095.
[15] Sabbaghan, M.; Sofalgar, P. Comb. Chem. High Throughput Screening 2015, 18, 901.
[16] Nagaraju, S.; Paplal, B.; Sathish, K.; Giri, S.; Kashinath, D. Tetrahedron Lett. 2017, 58, 4200.
[17] Chen, Z. W.; Zhang, N.; Wang, Z. H.; Su, W. K. Chin. Chem. Lett. 2013, 24, 199.
[18] Tröger, J. J. Prakt. Chem. 1887, 36, 225.
[19] Veale, E. B.; Frimannsson, D. O.; Lawler, M.; Gunnlaugsson, T. Org. Lett. 2009, 11, 4040.
[20] Li, W.; Michinobu, T. Chem. Phys. 2016, 217, 863.
[21] Li, M. Q. M.S. Thesis, Jiangsu Normal University, Xuzhou, 2018(in Chinese). (李明琪, 硕士论文, 江苏师范大学, 徐州, 2018.)
[22] Yuan, R.; Li, M. Q.; Xu, J. B.; Huang, S. Y.; Zhou, S. L.; Zhang, P.; Liu, J. J.; Wu, H. Tetrahedron 2016, 72, 4081.
[23] Lo, Q. A.; Sale, D.; Braddock, D. C.; Davies, R. P. ACS Catal. 2018, 8, 101.
[24] Malik, Q. M.; Ijaz, S.; Craig, D. C.; Try, A. C. Tetrahedron 2011, 67, 5798.



