[1] Li, P. T.; Zhang, Y. T., In Flora of China, Vol. 43, Eds.:Xu, L. R.; Huang, C. J., Science Press, Beijing, 1998, p. 2.
[2] Zamble, A.; Sahpaz, S.; Hennebelle, T.; Carato, P.; Bailleul, F. Chem. Biodiversity 2006, 3, 982.
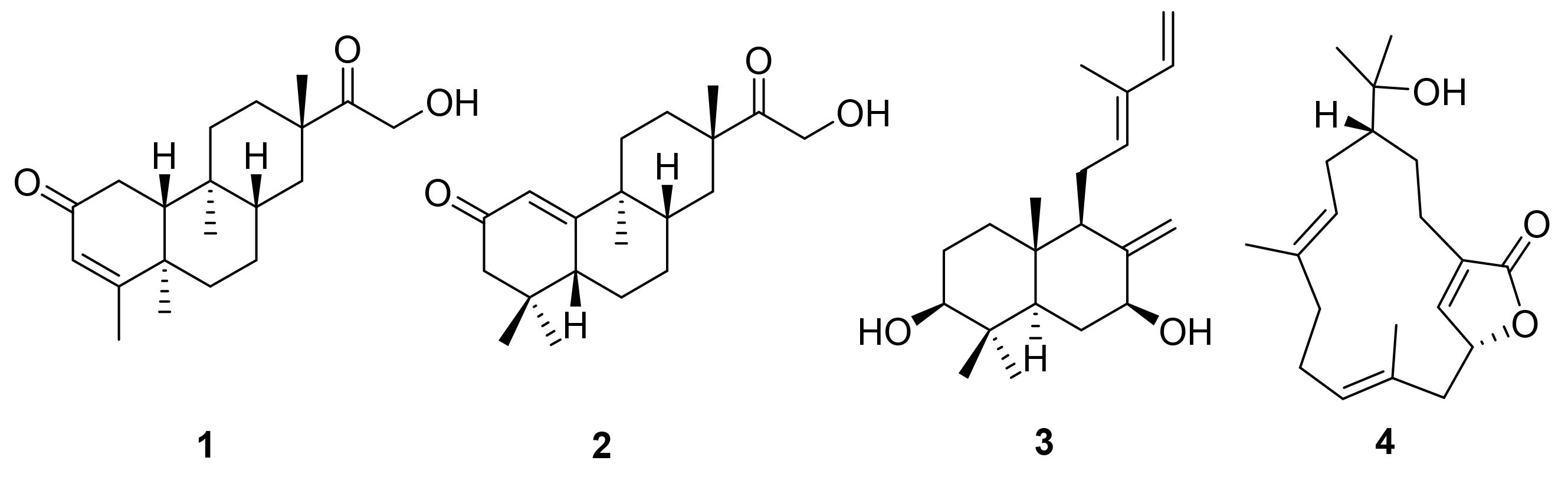
[3] Zamble, A.; Sahpaz, S.; Brunet, C.; Bailleul, F. Phytomedicine 2008, 15, 625.
[4] Roumy, V.; Hennebelle, T.; Zamble, A.; Yao, J. D.; Sahpaz, S.; Bailleul, F. Eur. J. Mass Spectrom. 2008, 14, 111.
[5] Zamble, A.; Martin-Nizard, F.; Sahpaz, S.; Reynaert, M.-L.; Staels, B.; Bordet, R.; Duriez, P.; Gressier, B.; Bailleul, F. Phytother. Res. 2009, 23, 892.
[6] Zamble, A.; Hennebelle, T.; Sahpaz, S.; Bailleul, F. Chem. Pharm. Bull. 2007, 55, 643.
[7] Akpanyung, E. O.; Ita, S. O.; Opara, K. A.; Davies, K. G.; Ndem, J. I.; Uwah, A. F. J. Med. Plants Res. 2013, 7, 2338.
[8] Ni, S.-J.; Li, J.; Li, M.-Y. Chem. Biodiversity 2018, 15, 1.
[9] Yang, Y.; Zhang, Y.; Liu, D.; Min, L.; Weber; Shao, B.; Lin, W.-H. Fitoterapia 2015, 103, 277.
[10] Bohlmann, F.; Knoll, K. H.; King, R. M.; Robinson, H. Phytochemistry 1979, 18, 1997.
[11] Ansell, S. M.; Pegel, K. H.; Taylor, D. A. H. Phytochemistry 1993, 32, 953.
[12] Jia, R.; Shi, Y.-H.; He, P.-M.; Guo, Y.-W. Chin. J. Nat. Med. 2010, 8, 422.
[13] Duh, C.-Y.; Wang, S.-K.; Weng, Y.-L.; Chiang, M.-Y.; Dai, C.-F. J. Nat. Prod. 1999, 62, 1518.
[14] Kijjoa, A.; Polonia, M. A.; Pinto, M. M. M.; Kitiratakarn, T.; Gedris, T.; Herz, W. Phytochemistry 1994, 37, 197.
[15] Mulholland, D. A.; Langat, M. K.; Crouch, N. R.; Coley, H. M.; Mutambi, E. M.; Nuzillard, J. M. Phytochemistry 2010, 71, 1381.
[16] Langat, M. K.; Crouch, N. R.; Smith, P. J.; Mulholland, D. A. J. Nat. Prod. 2011, 74, 2349.
[17] Kawakami, S.; Matsunami, K.; Otsuka, H.; Lhieochaiphant, D.; Lhieochaiphant, S. J. Nat. Med. 2013, 67, 410.
[18] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M.A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R. E.; Stratmann, O.; Yazyev, A. J.; Austin, R.; Cammi, C.; Pomelli, J. W.; Ochterski, R.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J., Gaussian 09, Rev. B.01, 2010, Gaussian Inc., Wallingford CT.
[19] Bruhn, T.; Schaumloeffel, A.; Hemberger, Y.; Bringmann, G. Chirality 2013, 25, 243.