

手性高价碘试剂诱导的烯烃不对称官能团化反应研究进展
收稿日期: 2020-06-09
修回日期: 2020-07-09
网络出版日期: 2020-08-11
基金资助
国家留学基金(201908620006); 甘肃省高等学校科学研究(2018B-091); 兰州石化职业技术学院教科研(JY2018-25)
Recent Advances in Asymmetric Functionalization of Olefins Induced by Chiral Hypervalent Iodine Reagents
Received date: 2020-06-09
Revised date: 2020-07-09
Online published: 2020-08-11
Supported by
the China Scholarship Council(201908620006); the Scientific Research Projects of Colleges and Universities in Gansu Province(2018B-091); the Teaching and Scientific Research Project of Lanzhou Petrochemical Polytechnic(JY2018-25)
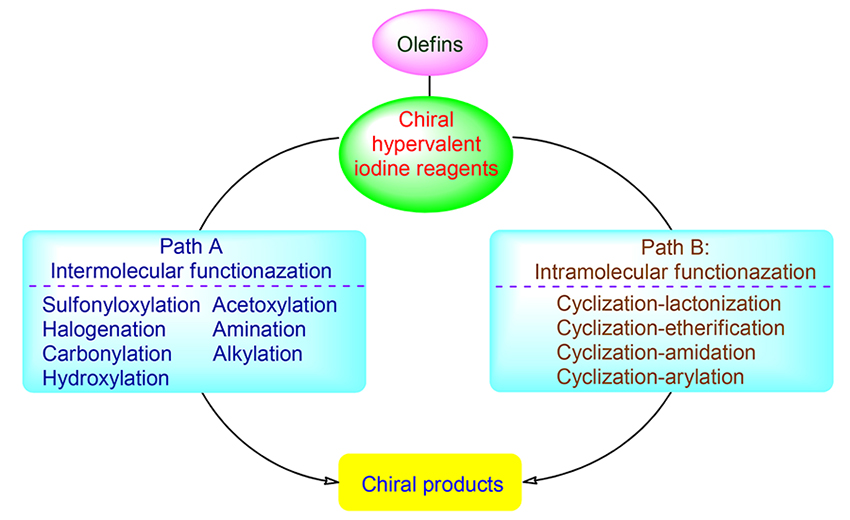
手性高价碘试剂诱导的烯烃官能团化是获得对映体富集的手性分子和具有生物活性天然产物的基本方法, 是不对称合成中一个崭新且富有成效的领域之一. 纵观20年来该领域的发展, 在手性高价碘诱导下, 一方面通过分子间的官能团化实现了双键的双磺酰氧基化、双乙酰氧基化、双卤化、双胺化、羟基化-磺酰氧基化、卤化-烷基化及羰基化等, 对映选择性地合成了各种官能团化的产物; 另一方面通过分子内的氧化内酯化、醚化、酰胺化、芳基化等反应, 对映选择性地合成了 γ-丁内酯、色满环、异色满环、噁唑烷酮、哌啶、吡咯烷、氮杂环丙烷等各种环状化合物, 为手性杂环化合物的合成提供了有效途径. 此外, 由于手性高价碘高效诱导烯烃发生各种官能团化, 提供了优秀的产率和对映选择性, 因此, 该方法学已被用于天然产物的全合成中. 就近二十年来手性高价碘试剂诱导的烯烃官能团化及其在天然产物全合成中的应用进行综述.
张怀远 , Thomas Wirth . 手性高价碘试剂诱导的烯烃不对称官能团化反应研究进展[J]. 有机化学, 2021 , 41(1) : 65 -70 . DOI: 10.6023/cjoc202006013
The functionalization of olefins induced by chiral hypervalent iodine reagents is a basic method for obtaining enantiomerically enriched chiral molecules and biologically active natural products. It is one of the new and promising fields in asymmetric synthesis. Throughout the past 20 years development in this area, on the one hand, stereoselective functionalizations of alkenes such as disulfonyloxylation, diacetoxylation, dihalogenation, diamination, hydroxylation-sulfonyloxylation and halogenation-alkylation and carbonylation have been successfully achieved with chiral hypervalent iodine reagents through intermolecular reactions, in which various functionalized products were enantioselectively obtained. On the other hand, many of hetero-cyclic compounds such as γ-butyrolactones, chromans, isochromans, oxazolidinones, piperidines, pyrrolidines and aziridines can be synthesized by intramolecular oxidative lactonization, etherification, amidation, arylation and other reactions, in which hypervalent iodine reagents can provide efficient methods for the synthesis of chiral heterocyclic compounds. In addition, various functionalization reactions of olefins mediated by chiral hypervalent iodine reagents can afford target compounds with excellent yield and enantioselectivity. Thus, this methodology has also been used in the total synthesis of natural products. The functionalization of olefins induced by chiral hypervalent iodine reagents and their applications in the total synthesis of natural products are reviewed.

| [1] | Noyori R. Asymmetric Catalysis in Organic Synthesis, John Wiley & Sons, Chichester, 1994. |
| [1] | Ojima I. Catalytic Asymmetric Synthesis, Wiley-VCH, Weinheim, 2000. |
| [1] | Beller M.; Bolm C. Transition Metals for Organic Synthesis, Vol. 1, Wiley-VCH, Weinheim, 2004. |
| [1] | (d) Lin G.-Q.; Sun X.-W.; Chen Y.-Q.; Li Y.-M.; Chan A.S.C. Chiral Synthesis :A Symmetric Reaction and its Application, Science Press, Beijing, 2013. |
| [1] | ( 林国强, 孙兴文, 陈耀全, 李月明, 陈新滋, 手性合成: 不对称反应及其应用, 科学出版社, 北京, 2013. ). |
| [1] | (e) Carney J.R.; Dillon B.R.; Thomas S.P. Eur. J. Org. Chem. 2016, 3912. |
| [1] | (f) Romero N.A.; Nicewicz D.A. Chem. Rev. 2016, 116, 10075. |
| [1] | (g) Vekariya R.L. J. Mol. Liq. 2017, 227, 44. |
| [1] | (h) Srivastava V.; Singh P.P. RSC Adv. 2017, 7, 31377. |
| [1] | (i) Patel N.; Sood R.; Bharatam P.V. Chem. Rev. 2018, 118, 8770. |
| [1] | (j) Trowbridge A.; Walton S.M.; Gaun M.J. Chem. Rev. 2020, 120, 2613. |
| [1] | (k) Sheldon R.A.; Brady D.; Bode M.L. Chem. Sci. 2020, 11, 2587. |
| [2] | Jacobsen E.N.; Pfaltz A.; Yamamoto H. Comprehensive Asymmetric Catalysis, Springer, Berlin, 2000. |
| [2] | (b) Blaser H.-U.; Federsel H.-J. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions, Wiley-VCH, Weinheim, 2004. |
| [2] | (c) Krautwald S.; Carreira E.M. J. Am. Chem. Soc. 2017, 139, 5627. |
| [2] | (d) Bhattacharjee S.; Khan M.I.; Li X.; Zhu Q.-L.; Wu X.-T. Catalysts 2018, 8, 120. |
| [2] | (e) Lin Q.; Li L.; Luo S. Chem. -Eur. J. 2019, 25, 10033. |
| [2] | (f) Shaw S.; White J.D. Chem. Rev. 2019, 119, 9381. |
| [2] | (g) Wrzeszcz Z.; Siedlecka R. Molecules 2020, 25, 330. |
| [3] | Berkessel A.; Go?ger H. Asymmetric Organocatalysis :from Biomimetic Concepts to Applications in Asymmetric Synthesis, Wiley-VCH, Weinheim, 2005. |
| [3] | (b) Dalko P.I. Enantioselective Organocatalysis :Reactions and Experimental Procedures, Wiley-VCH, Weinheim, 2007. |
| [3] | (c) Erkkilä A.; Majander I.; Pihko P.M. Chem. Rev. 2007, 107, 5416. |
| [3] | (d) MacMillan D.W.C. Nature 2008, 455, 304. |
| [3] | (e) Barbas C.F. Angew. Chem., Int. Ed. 2008, 47, 42. |
| [3] | (f) Melchiorre P.; Marigo M.; Carlone A.; Bartoli G. Angew. Chem., Int. Ed. 2008, 47, 6138. |
| [3] | (g) Sun Y.-L.; Wei Y.; Shi M. ChemCatChem 2017, 9, 718. |
| [3] | (h) Nguyen T.N.; Chen P.-A.; Setthakarn K.; May J.A. Molecules 2018, 23, 2317. |
| [4] | (a) Adolfsson H. Angew. Chem., Int. Ed. 2005, 44, 3340. |
| [4] | (b) Ouellet S.G.; Walji A.M.; MacMillan D.W.C. Acc. Chem. Res. 2007, 40, 1327. |
| [4] | (c) Connon S.J. Org. Biomol. Chem. 2007, 5, 3407. |
| [4] | (d) Wang Z.; Jiang Z. Asian J. Chem. 2010, 22, 4141. |
| [4] | (e) de Vries, J.G.; Mršić, N. Catal. Sci. Technol. 2011, 1, 727. |
| [4] | (f) Rueping M.; Dufour J.; Schoepke F.R. Green Chem. 2011, 13, 1084. |
| [4] | (g) Phillips A.M.F.; Pombeiro A.J.L. Org. Biomol. Chem. 2017, 15, 2307. |
| [5] | (a) Wirth T. Hypervalent Iodine Chemistry in Topics in Current Chemistry, Vol.373, Springer, Switzerland, 2016. |
| [5] | (b) Zhdankin V.V.; Stang P.J. Chem. Rev. 2002, 102, 2523. |
| [5] | (c) Wirth T. Angew. Chem., Int. Ed. 2005, 44, 3656. |
| [5] | (d) Uyanik M.; Ishihara K. Chem. Commun. 2009, 2086. |
| [5] | (e) Merritt E.A.; Olofsson B. Angew. Chem., Int. Ed. 2009, 48, 9052. |
| [5] | (f) Zhdankin V.V. J. Org. Chem. 20 11, 76, 1185. |
| [5] | (g) González D.F.; Benfatti F.; Waser J. ChemCatChem 2012, 4, 955. |
| [5] | Singh F.V.; Wirth T. Synthesis 2013, 45, 2499. |
| [5] | (i) Zheng Z.S.; Zhang-Negrerie D.; Du Y.F.; Zhao K. Sci. China :Chem. 2014, 57, 189. |
| [5] | (j) Yoshimura A.; Zhdankin V.V. Chem. Rev. 2016, 116, 3328. |
| [5] | (k) Yoshimura A.; Yusubov M.S.; Zhdankin V.V. Org. Biomol. Chem. 2016, 14, 4771. |
| [5] | (l) Zhang H.; Tang R.; Wu J.; Hu Y. Chemistry 2018, 681. |
| [5] | ( 张怀远, 唐蓉萍, 伍家卫, 胡雨来, 化学通报, 2018, 681.). |
| [5] | (m) Reddy Kandimalla, S.; Prathima Parvathaneni, S.; Sabitha, G.; Subba Reddy, B.V. Eur. J. Org. Chem. 2019, 1687. |
| [5] | (n) Parra A. Chem. Rev. 2019, 119, 12033. |
| [5] | (o) Zhang H.; Tang R.; Shi X.; Xie L.; Wu J. Chin. J. Org. Chem. 2019, 39, 1837. |
| [5] | ( 张怀远, 唐蓉萍, 石星丽, 颉林, 伍家卫, 有机化学, 2019, 39, 1837.). |
| [5] | (p) Cai Q.; Ma H. Acta Chim. Sinica 2019, 77, 213. |
| [5] | ( 蔡倩, 马浩文, 化学学报, 2019, 77, 213.). |
| [5] | (q) Liu D.; He J.; Zhang C. Univ. Chem. 2019, 34, 1. |
| [5] | ( 刘丹, 贺家豪, 张弛, 大学化学, 2019, 34, 1.). |
| [6] | (a) Imamoto T.; Koto H. Chem. Lett. 1986, 15, 967. |
| [6] | (b) Ray III, D.G.; Koser, G.F. J. Am. Chem. Soc. 1990, 112, 5672. |
| [6] | (c) Xia M.; Chen Z.-C. Synth. Commun. 1997, 27, 1315. |
| [6] | (d) Tohma H.; Takizawa S.; Watanabe H.; Fukuoka Y.; Maegawa T.; Kita Y. J. Org. Chem. 1999, 64, 3519. |
| [6] | (e) Ladziata U.; Carlson J.; Zhdankin V.V. Tetrahedron Lett. 2006, 47, 6301. |
| [7] | (a) Ochiai M.; Kitagawa Y.; Takayama N.; Takaoka Y.; Shiro M. J. Am. Chem. Soc. 1999, 121, 9233. |
| [7] | (b) Companys S.; Peixoto P.A.; Bosset C.; Chassaing S.; Miqueu K.; Sotiropoulos J.-M.; Pouységu L.; Quideau S. Chem. -Eur. J. 2017, 23, 13309. |
| [8] | (a) Yu J.; Cui J.; Hou X.-S.; Liu S.-S.; Gao W.-C.; Jiang S.; Tian J.; Zhang C. Tetrahedron :Asymmetry 2011, 22, 2039. |
| [8] | (b) Mizar P.; Wirth T. Angew. Chem., Int. Ed. 2 014, 53, 5993. |
| [8] | (c) Suzuki S.; Kamo T.; Fukushi K.; Hiramatsu T.; Tokunaga E.; Dohi T.; Kita Y.; Shibata N. Chem. Sci. 2014, 5, 2754. |
| [8] | Brenet S.; Minozzi C.; Clarens B.; Amiri L.; Berthiol F. Synthesis 2015, 47, 3859. |
| [8] | (e) Basdevant B.; Legault C.Y. Org. Lett. 2015, 17, 4918. |
| [8] | (f) Cao Y.; Zhang X.; Lin G.; Zhang-Negrerie D.; Du Y. Org. Lett. 2016, 18, 5580. |
| [8] | (g) Pluta R.; Krach P.E.; Cavallo L.; Falivene L.; Rueping M. ACS Catal. 2018, 8, 2582. |
| [8] | (h) Wang Y.; Yuan H.; Lu H.; Zheng W.-H. Org. Lett. 2018, 20, 2555. |
| [9] | (a) Dohi T.; Maruyama A.; Takenaga N.; Senami K.; Minamitsuji Y.; Fujioka H.; Caemmerer S.B.; Kita Y. Angew. Chem., Int. Ed. 2008, 47, 3787. |
| [9] | (b) Dohi T.; Takenaga N.; Nakae T.; Toyoda Y.; Yamasaki Y.; Shiro M.; Fujioka H.; Maruyama A.; Kita Y. J. Am. Chem. Soc. 2013, 135, 4558. |
| [9] | (c) Uyanik M.; Yasui T.; Ishihara K. Angew. Chem., Int. Ed. 2013, 52, 9215. |
| [9] | (d) Bosset C.; Coffinier R.; Peixoto P.A.; El Assal M.; Miqueu K.; Sotiropoulos J.M.; Pouységu L.; Quideau S. Angew. Chem., Int. Ed. 2014, 53, 9860. |
| [9] | (e) Uyanik M.; Sasakura N.; Mizuno M.; Ishihara K. ACS Catal. 2017, 7, 872. |
| [9] | (f) Dohi T.; Sasa H.; Miyazaki K.; Fujitake M.; Takenaga N.; Kita Y. J. Org. Chem. 2017, 82, 11954. |
| [9] | (g) Hashimoto T.; Shimazaki Y.; Omatsu Y.; Maruoka K. Angew. Chem., Int. Ed. 2018, 57, 7200. |
| [9] | (h) Antien K.; Pouységu L.; Deffieux D.; Massip S.; Peixoto P.A.; Quideau S. Chem. -Eur. J. 2019, 25, 2852. |
| [10] | Malmedy F.; Wirth T. Chem. -Eur. J. 2016, 22, 16072. |
| [11] | (a) Castellanos, A; Fletcher, S.P.Chem. -Eur. J. 2011, 17, 5766. |
| [11] | (b) Verendel J.J.; Pàmies O.; Diéguez M.; Andersson P.G. Chem. Rev. 2014, 114, 2130. |
| [11] | (c) Coombs J.R.; Morken J.P. Angew. Chem., Int. Ed. 2016, 55, 2636. |
| [11] | (d) Margarita C.; Andersson P.G. J. Am. Chem. Soc. 2017, 139, 1346. |
| [11] | (e) Kraft S.; Ryan K.; Kargbo R.B. J. Am. Chem. Soc. 2017, 139, 11630. |
| [11] | (f) Fu X.; Zhao W. Chin. J. Org. Chem. 2019, 39, 625. |
| [11] | ( 付晓飞, 赵文献, 有机化学, 2019, 39, 625.). |
| [12] | Wirth T.; Hirt U.H. Tetrahedron : Asymmetry 1997, 8, 23. |
| [13] | Hirt U.H.; Spingler B.; Wirth T. J. Org. Chem. 1998, 63, 7674. |
| [14] | Hirt U.H.; Schuster M.F.H.; French A.N.; Wiest O.G.; Wirth T. Eur. J. Org. Chem. 20 01, 1569. |
| [15] | Koposov A.Y.; Boyarskikh V.V.; Zhdankin V.V. Org. Lett. 2004, 6, 3613. |
| [16] | (a) Ngatimin M.; Gartshore C.J.; Kindler J.P.; Naidu S.; Lupton D.W. Tetrahedron Lett. 2009, 50, 6008. |
| [16] | (b) Boppisetti J.K.; Birman V.B. Org. Lett. 2009, 11, 1221. |
| [17] | Fujita M.; Wakita M.; Sugimura T. Chem. Commun. 2 011, 47, 3983. |
| [18] | Shimogaki M.; Fujita M.; Sugimura T. Molecules 2015, 20, 17041. |
| [19] | Fujita M.; Miura K.; Sugimura T. Beilstein J. Org. Chem. 2018, 14, 659. |
| [20] | Ro?ben C.; Souto J.A.; González Y.; Lishchynskyi A.; Muñiz K. Angew. Chem., Int. Ed. 2011, 50, 9478. |
| [21] | Ro?ben C.; Souto J.A.; Escudero-Adán E.C.; Muñiz K. Org. Lett. 2013, 15, 1008. |
| [22] | Farid U.; Malmedy F.; Claveau R.; Albers L.; Wirth T. Angew. Chem., Int. Ed. 2013, 52, 7018. |
| [23] | Brown M.; Kumar R.; Rehbein J.; Wirth T. Chem. -Eur. J. 2016, 22, 4030. |
| [24] | Qurban J.; Elsherbini M.; Wirth T. J. Org. Chem. 2017, 82, 11872. |
| [25] | Hokamp T.; Wirth T. J. Org. Chem. 2019, 84, 8674. |
| [26] | Zhang D.-Y.; Zhang Y.; Wu H.; Gong L.-Z. Angew. Chem., Int. Ed. 2019, 58, 7450. |
| [27] | Ahmad A.; Silva Jr, L.F. J. Org. Chem. 2016, 81, 2174. |
| [28] | Jobin-Des Lauriers, A.; Legault, C.Y. Org. Lett. 2016, 18, 108. |
| [29] | Haubenreisser S.; Wo?ste T.H.; Martínez C.; Ishihara K.; Mun?iz K. Angew. Chem., Int. Ed. 2016, 55, 413. |
| [30] | Wo?ste T.H.; Mun?iz K. Synthesis 2016, 48, 816. |
| [31] | Molnár I.G.; Gilmour R. J. Am. Chem. Soc. 2016, 138, 5004. |
| [32] | Banik S.M.; Medley J.W.; Jacobsen E.N. J. Am. Chem. Soc. 2016, 138, 5000. |
| [33] | Haj M.K.; Banik S.M.; Jacobsen E.N. Org. Lett. 2019, 21, 4919. |
| [34] | Banik S.M.; Medley J.W.; Jacobsen E.N. Science 2016, 353, 51. |
| [35] | Mun?iz K.; Barreiro L.; Romero R.M.; Martínez C. J. Am. Chem. Soc. 2017, 139, 4354. |
| [36] | Sreenithya A.; Hadad C.M.; Sunoj R.B. Chem. Sci. 2019, 10, 7082. |
| [37] | Boye A.C.; Meyer D.; Ingison C.K.; French A.N.; Wirth T. Org. Lett. 2003, 5, 2157. |
| [38] | Fujita M.; Okuno S.; Lee H.J.; Sugimura T.; Okuyama T. Tetrahedron Lett. 2007, 48, 8691. |
| [39] | Fujita M.; Lee H.J.; Sugimura T.; Okuyama T. Chem. Commun. 2007, 1139. |
| [40] | Fujita M.; Yoshida Y.; Miyata K.; Wakisaka A.; Sugimura T. Angew. Chem., Int. Ed. 2010, 49, 7068. |
| [41] | (a) Fujita M.; Mori K.; Shimogaki M.; Sugimura T. Org. Lett. 2012, 14, 1294. |
| [41] | (b) Shimogaki M.; Fujita M.; Sugimura T. Eur. J. Org. Chem. 2013, 7128. |
| [42] | Deng Q.-H.; Wang J.-C.; Xu Z.-J.; Zhou C.-Y.; Che C.-M. Synthesis 2011, 2959. |
| [43] | Farid U.; Wirth T. Angew. Chem., Int. Ed. 2012, 51, 3462. |
| [44] | Kong W.; Feige P.; de Haro T.; Nevado C. Angew. Chem., Int. Ed. 2013, 52, 2469. |
| [45] | Mizar P.; Laverny A.; El-Sherbini M.; Farid U.; Brown M.; Malmedy F.; Wirth T. Chem. -Eur. J. 2014, 20, 9910. |
| [46] | Alhalib A.; Kamouka S.; Moran W.J. Org. Lett. 2015, 17, 1453. |
| [47] | Mizar P.; Niebuhr R.; Hutchings M.; Farooq U.; Wirth T. Chem. -Eur. J. 2016, 22, 1614. |
| [48] | Shimogaki M.; Fujita M.; Sugimura T. Angew. Chem., Int. Ed. 2016, 55, 15797. |
| [49] | Shimogaki M.; Fujita M.; Sugimura T. J. Org. Chem. 2017, 82, 11836. |
| [50] | Woerly E.M.; Banik S.M.; Jacobsen E.N. J. Am. Chem. Soc. 2016, 138, 13858. |
| [51] | Geary G.C.; Hope E.G.; Stuart A.M. Angew. Chem., Int. Ed. 2015, 54, 14911. |
| [52] | Mennie K.M.; Banik S.M.; Reichert E.C.; Jacobsen E.N. J. Am. Chem. Soc. 2018, 140, 4797. |
| [53] | Gelis C.; Dumoulin A.; Bekkaye M.; Neuville L.; Masson G. Org. Lett. 2017, 19, 278. |
| [54] | (a) Guimarães K.G.; Dias de Souza Filho, J.; Rennó dos Mares-Guia, T.; Castro Braga, F. Phytochemistry 2008, 69, 439. |
| [54] | (b) Endringer D.C.; Guimarães K.G.; Kondratyuk T.P.; Pezzuto J.M.; Braga F.C. J. Nat. Prod. 2008, 71, 1082. |
| [55] | Fujita M.; Mori K.; Shimogaki M.; Sugimura T. RSC Adv. 2013, 3, 17717. |
| [56] | Takesue T.; Fujita M.; Sugimura T.; Akutsu H. Org. Lett. 2014, 16, 4634. |
| [57] | (a) Garson M.J.; Staunton J.; Jones P.G. J. Chem. Soc., Perkin Trans. 1 1984, 1021. |
| [57] | (b) Krohn K.; Kock I.; Elsässer B.; Flörke U.; Schulz B.; Draeger S.; Pescitelli G.; Antus S.; Kurtán T. Eur. J. Org. Chem. 2007, 1123. |
/
| 〈 |
|
〉 |