

炔醇分子内环化促发的串联反应在螺杂环化合物合成中的应用
收稿日期: 2020-06-23
修回日期: 2020-07-28
网络出版日期: 2020-08-19
基金资助
国家自然科学基金(21602207); 郑州轻工业大学博士科研基金(2014BSJJ009); 郑州轻工业大学青年骨干教师(2019XGGJS010)
Application of Cascade Reactions in the Synthesis of Sprio-hetero- cycles Initiated by Intramolecular Cyclization of Alkynols
Received date: 2020-06-23
Revised date: 2020-07-28
Online published: 2020-08-19
Supported by
the National Natural Science Foundation of China(21602207); the Doctoral Research Fund of Zhengzhou University of Light Industry(2014BSJJ009); the Young Backbone Teachers’ Fund of Zhengzhou University of Light Industry(2019XGGJS010)
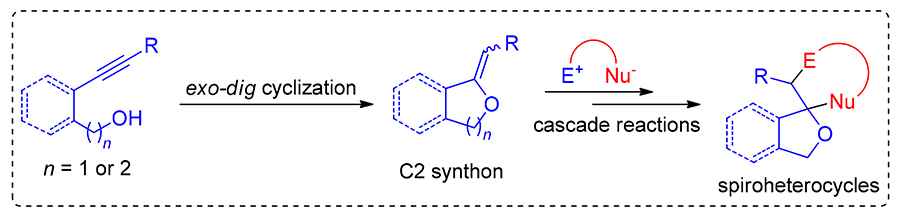
螺杂环化合物独特的立体结构和丰富的理化特性, 激发了研究工作者对其高效合成方法的持续关注. 炔醇在过渡金属作用下经exo-dig式分子内环化可原位形成环外烯醇醚, 其作为一类高活性的C2合成子, 能够与同时具有亲电和亲核特性的“双亲性底物”发生串联反应, 实现螺杂环骨架的快速构建. 综述了近年来炔醇分子内环化促发的串联反应在螺杂环化合物合成中的应用进展, 以期激发更多相关研究工作的设计与报道. 按照参与螺环构建的“双亲性底物”原子数目的不同进行分类, 重点阐述了反应采用的催化体系和反应机制, 分析了目前该领域存在的挑战, 并对未来的发展进行了展望.
余述燕 , 高丽宏 , 闫溢哲 , 尹志刚 , 商永嘉 . 炔醇分子内环化促发的串联反应在螺杂环化合物合成中的应用[J]. 有机化学, 2021 , 41(2) : 582 -593 . DOI: 10.6023/cjoc202006050
Due to their unique stereoscopic structure and rich physical and chemical properties, spiro heterocyclic compounds have aroused the continuous attention of researchers on their efficient synthesis methods. The exo-cyclic enol ethers generated in situ by exo-dig cyclization of alkynols under the promotion of transition metals could serve as C2 synthons to react with various "amphiphilic substrates" through cascade reactions mode. These cascade reactions could afford spiro heterocyclic skeletons in highly efficient and direct manners. In this paper, the application progress of cascade reactions in the synthesis of sprio-heterocycles initiated by intramolecular cyclization of alkynols is reviewed, which aims to stimulate the disclosure of more related research work. These work is elaborated according to the different atom numbers of the "amphiphilic substrates" involved in the construction of the spiro framworks. The catalytic system and reaction mechanism are mainly described, the challenges in this field are analyzed, and the future development is also put forward.

Key words: sprio-heterocycles; alkynols; cascade reactions; exo-cyclic enol ether
| [1] | Krapcho, A.P. Synthesis 1974, 383. |
| [1] | Lovering, F.; Bikker, J.; Humblet, C. J. Med. Chem. 2009, 52,6752. |
| [1] | Nai, G.X.; Dai, R.Q. Technol. Dev. Chem. Ind. 2012, 41, 6. (in Chinese) |
| [1] | 赖谷仙, 戴日强, 化工技术与开发,2012, 41, 6.). |
| [1] | Zou, L.; Wang, C.S.; Liang, B. New Chem. Mater. 2013, 41, 12. (in Chinese) |
| [1] | 邹琳, 王长松, 梁兵, 化工新型材料,2013, 41, 12.). |
| [1] | Fan, K.Q.; Wang, X.B.; Ma, Y.P.; Yang, H.R.; Han, G.L.; Zhou, L.M.; Fang, S.M. New J. Chem. 2020, 44, 8351. |
| [2] | Wei, R.B.; Liang, Y. Chin. J. Org. Chem. 2008, 28, 1501. (in Chinese) |
| [2] | 魏荣宝, 梁娅, 有机化学,2008, 28, 1501.). |
| [2] | Li, F.F.; Wang, L.; Chen, S.Y.; Zhu, H.M.; Ouyang, G.P. Chem. Res. Appl. 2012, 24, 1181. (in Chinese) |
| [2] | 李飞飞, 王磊, 陈舒忆, 朱红梅, 欧阳贵平, 化学研究与应用,2012, 24, 1181.). |
| [2] | Wei, R.B.; Zhang, D.W.; Liu, B.; Liu, Y.; Jia, C.X. Chin. J. Org. Chem. 2009, 29, 517. (in Chinese) |
| [2] | 魏荣宝, 张大为, 刘博, 刘洋, 李文丽, 贾辰熙, 有机化学,2009, 29, 517.). |
| [2] | Michael, J.B. Carbon 1997, 35, 1. |
| [3] | Rios, R. Chem. Soc. Rev. 2012, 41, 1060. |
| [4] | Kotha, S.; Panguluri, N.R.; Ali, R. Eur. J. Org. Chem. 2017, 5316. |
| [5] | Li, X.F. Ph. D. Dissertation, Tianjing University, Tianjing, 2003. (in Chinese) |
| [5] | 李筱芳, 博士论文, 天津大学, 天津,2003. ). |
| [6] | Alcaide, B.; Almendros, P.; Alonso, J.M. Molecules 2011, 16, 7815. |
| [6] | Zeng, X.M. Chem. Rev. 2013, 113, 6864. |
| [6] | Alcaide, B.; Almendros, P. Acc. Chem. Res. 2014, 47, 939. |
| [7] | Solomon, V.R.; Lee, H. Curr. Med. Chem. 2011, 18, 1488. |
| [7] | Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. Eur. J. Med. Chem. 2015, 97, 871. |
| [8] | Kouznetsov, V.V.; Mendez, L. Y. V.; Gomez, C. M. M. Curr. Org. Chem. 2005, 9, 141. |
| [8] | Kouznetsov, V.V. Tetrahedron 2009, 65, 2721. |
| [8] | Fochi, M.; Caruana, L.; Bernardi, L. Synthesis 2014, 46, 135. |
| [9] | Barluenga, J.; Mendoza, A.; Rodríguez, F.; Fa?anás, F.J. Angew. Chem. Int. Ed. 2008, 47, 7044. |
| [10] | Wang, L.; Liu, L.Y.; Chang, W.X.; Li, J. J. Org. Chem. 2018, 83, 7799. |
| [11] | Aho, J.E.; Pihko, P.M.; Rissa, T.K. Chem. Rev. 2005, 105, 4406. |
| [12] | Uckun, F.M.; Mao. C.; Vassilev, A.O.; Huang, H.; Jan, S.T. Bioorg. Med. Chem. Lett. 2000, 10, 541. |
| [12] | Barun, O.; Kumar, K.; Sommer, S.; Langerak, A.; Mayer, T.U.; Muller, O.; Waldman, H. Eur. J. Org. Chem. 2005, 4773. |
| [13] | Mead, K.T.; Brewer, B.N. Curr. Org. Chem. 2003, 7, 227. |
| [14] | Barluenga, J.; Mendoza, A.; Rodríguez, F.; Fa?anás, F.J. Angew. Chem. Int. Ed. 2009, 48, 1644. |
| [15] | Wu, H.; He, Y.P.; Gong, L.Z. Org. Lett. 2013, 15, 460. |
| [16] | Liang, M. M.S. Thesis, Shangdong University, Jinan, 2018. (in Chinese) |
| [16] | 梁曼, 硕士论文, 山东大学, 济南,2018. ). |
| [17] | Wang, X.H.; Dong, S.L.; Yao, Z.L.; Feng, L.; Daka, P.; Wang, H.; Xu, Z.H. Org. Lett. 2014, 16, 22. |
| [18] | Li, J.; Lin, L.; Hu, B.; Lian, X.; Wang, G.; Liu, X; Feng, X. Angew. Chem. Int. Ed. 2016, 55, 6075. |
| [19] | Gong, J.; Wan, Q.; Kang, Q. Adv. Synth. Catal. 2018, 360, 4031. |
| [20] | Willis, N.J.; Bray, C.D. Chem .- Eur. J. 2012, 18, 9160. |
| [20] | Bai, W.J.; David, J.G.; Feng, Z.G.; Weaver, M. G. Wu, K. L.; Pettus, T. R. R. Acc. Chem. Res. 2014, 47, 3655. |
| [20] | Caruana, L.; Fochi, M.; Bernardi, L. Molecules 2015, 20, 11733;. |
| [20] | Ai, W.; Liao, D.; Lei, X. Chin. J. Org. Chem. 2015, 35, 1615. (in Chinese) |
| [20] | 艾文英, 廖道红, 雷晓光, 有机化学,2015, 35, 1615.). |
| [21] | Liang, M.; Zhang, S.; Jia, J.; Tung, C.H.; Wang, J.W.; Xu, Z.H. Org. Lett. 2017, 19, 2526. |
| [22] | Wang, C.S.; Cheng, Y.C.; Zhou, J.; Mei, G.J.; Wang, S.L.; Shi, F. J. Org. Chem. 2018, 83, 13861. |
| [23] | Gharpure, S.J.; Nanda, S.K.; Shelke, Y.G. Chem .- Eur. J. 2017, 23, 10007. |
| [24] | Stierle, A.A.; Stierle, D.B.; Kelly, K. J. Org. Chem. 2006, 71, 5357. |
| [25] | Arto, T.; Santa-Mar?a, I.S.; Chiara, M.D.; Fa?anás, F.J.; Rodríguez, F. Eur. J. Org. Chem. 2016, 5876. |
| [26] | Elsharif, A.M. Orient. J. Chem. 2019, 35, 658. |
| [26] | de Fatima, A.; Braga, T.C.; Neto, L. D. S.; Terra, B.S.; Oliveira, B. G. F.; da Silva, D.L.; Modolo, L.V. J. Adv. Res. 2015, 6, 363. |
| [26] | Wang, B.Z.; Xu, X.Y.; Tao, Y.C.; Ke, S.Y.; Qian, X.H.; Li, Z. Chin. J. Pestic. Sci. 2010, 12, 429. (in Chinese) |
| [26] | 王宝珠, 徐晓勇, 陶玉成, 柯少勇, 钱旭红, 李忠, 农药学学报,2010, 12, 429.). |
| [27] | Kappe, C.O. Acc. Chem. Res. 2000, 33, 879. |
| [27] | Gong, L.Z.; Chen, X.H.; Xu, X.Y. Chem .- Eur. J. 2007, 13, 8920. |
| [27] | Heravi, M.M.; Moradi, R.; Mohammadkhani, L.; Moradi, B. Mol. Diversity 2018, 22, 751. |
| [28] | Yu, S.Y.; Wu, J.X.; Lan, H.B.; Gao, L.H.; Qian, H.Y.; Fan, K.Q.; Yin, Z.G. Org. Lett. 2020, 22, 102. |
| [28] | Yu, S.Y.; Gao, L.H.; Lan, H.B.; Qian, H.Y.; Yin, Z.G.; Shang, Y.J. Chin. J. Org. Chem. 2020, 40, 2714. (in Chinese) |
| [28] | 余述燕, 高丽宏, 兰宏兵, 钱恒玉, 尹志刚, 商永嘉, 有机化学,2020, 40, 2714.). |
| [28] | Yu, S.Y.; Wu, J.X.; Lan, H.B.; Xu, H.W.; Shi, X.F.; Zhu, X.W.; Yin, Z.G. RSC Adv. 2018, 8, 33968. |
| [28] | Yu, S.Y.; Gao, L.H.; Wu, J.X.; Lan, H.B.; Ma, Y.; Yin, Z.G. Chem. Pap. 2020, 74, 3303. |
| [28] | Yu, S.Y.; Wu, J.X.; He, X.W.; Shang, Y.J. Appl. Organomet. Chem. 2018, 32, e4156. |
| [28] | Yu, S.Y.; Zhang, Z.Q.; Yu, Z.Y.; Shang, Y.J. Appl. Organomet. Chem. 2014, 28, 657. |
| [29] | Yao, H.L.; Wang, J.; Tong, R.B. Chem. Rev. 2017, 17, 1109. |
| [29] | Wang, J.; Tong, R.B. J. Org. Chem. 2016, 81, 4325. |
| [30] | Cala, L.; Mendoza, A.; Fa?anás, F.J.; Rodríguez, F. Chem. Commun. 2013, 49, 2715. |
| [31] | Kambale, D.A.; Thorat, S.S.; Pratapure, M.S.; Gonnade, R.G.; Kontham, R. Chem. Commun. 2017, 53, 6641. |
| [32] | Teng, Q.; Qi, J.L.; Zhou, L.; Xu, Z.H.; Tung, C.H. Org. Chem. Front. 2018, 5, 990. |
| [33] | Jackson, S.K.; Banfield, S.C, Kerr, M.A. Org. Lett. 2005, 7, 1215. |
| [33] | Uliana, M.P.; Servilha, B.M.; Alexopoulos, O.; de Oliveira, K.T.; Tormena, C.F.; Ferreira, M. A. B.; Brocksom, T.J. Tetrahedron 2014, 70, 6963. |
| [34] | Liao, L.H.; Shu, C.; Zhang, M.M.; Liao, Y.J.; Hu, X.Y.; Zhang, Y.H.; Wu, Z.J.; Yuan, W.C.; Zhang, X.M. Angew. Chem. Int. Ed. 2014, 53, 10471. |
| [34] | Sun. X.X.; Zhang, H.H.; Li, G.H.; Meng, L.; Shi, F. Chem. Commun. 2016, 52, 2968. |
| [34] | Shu, C.; Liao, L.H; Liao. Y. J.; Hu, X.Y.; Zhang, Y.H.; Yuan, W.C.; Zhang, X.M. Eur. J. Org. Chem. 2014, 4467. |
| [35] | Jasper, A.; Schepmann, D.; Lehmkuhl, K.; Vela, J.M.; Buschmann, H.; Holenz, J.; Wünsch, B. Eur. J. Med. Chem. 2012, 53, 327. |
| [35] | Strachan, J.; Farias, J.J.; Zhang, J.; Caldwell, W.S.; Bhatti, B.S. Bioorg. Med. Chem. Lett. 2012, 22, 5089. |
| [36] | Sweeney, J.B. Chem. Soc. Rev. 2002, 31, 247. |
| [37] | Huisgen, R.; Scheer, W.; M?der, H. Angew. Chem. Int. Ed. 1969, 8, 602. |
| [38] | Lu, P.F. Tetrahedron 2010, 66, 2549. |
| [38] | Pineschi, M. Eur. J. Org. Chem. 2006, 4979. |
| [38] | Schneider, C. Angew. Chem. Int. Ed. 2009, 48, 2082. |
| [39] | Wang, B.; Liang, M.; Tang, J.; Deng, Y.T.; Zhao, J.H.; Sun, H.; Tung, C.H.; Jia, J.; Xu, Z.H. Org. Lett. 2016, 18, 4614. |
| [40] | Wagner, B.; Hiller, W.; Ohno, H.; Krause, N. Org. Biomol. Chem. 2016, 14, 1579. |
| [41] | Sun, W.S.; Zhu, G.M.; Wu, C.Y.; Hong, L.; Wang, R. Chem .- Eur. J. 2012, 18, 6737. |
| [41] | Singh, K.; Pramanik, S.; Hamlin, T.A.; Mondal, B.; Das, D.; Saha, J. Chem. Commun. 2019, 55, 7069. |
| [42] | Kusama, H.; Ebisawa, M.; Funama, H.; Iwasawa, N. J. Am. Chem. Soc. 2009, 131, 45, 16352. |
| [43] | Yao, T.; Zhang, X.; Larock, R.C. J. Am. Chem. Soc. 2004, 126, 40, 11164. |
| [44] | Patil, N.T.; Wu, H.; Yamamoto, Y. J. Org. Chem. 2005, 70, 4531. |
| [44] | Oh, C.H.; Reddy, V.R.; Kim, A.; Rhim, C.Y. Tetrahedron Lett. 2006, 47, 5307. |
| [44] | Liu, Y.H.; Zhou, S. Org. Lett. 2005, 6, 4609. |
| [44] | Liu, F. Ph. D. Dissertation, East China Normal University, Shanghai,2013. (in Chinese) |
| [44] | 刘锋, 博士论文, 华东师范大学, 上海,2013. ). |
| [44] | Di, X.Y. Ph. D. Dissertation, East China Normal University, Shanghai,2019. (in Chinese) |
| [44] | 底晓煜, 博士论文, 华东师范大学, 上海,2019. ). |
| [45] | Qi, J.L.; Teng, Q.; Thirupathi, N.; Tung, C.H.; Xu, Z.H. Org. Lett. 2019, 21, 692. |
| [46] | Barluenga, J.; Calleja, J.; Mendoza, A.; Rodrígue, F.; Fa?anás, F J. Chem .- Eur. J. 2010, 16, 7110. |
| [47] | Wang, X.; Yao, Z.; Dong, S.; Wei, F.; Wang, H.; Xu, Z.H. Org. Lett. 2013, 15, 2234. |
/
| 〈 |
|
〉 |