

铜催化氧化和Aza-Diels-Alder反应三组分合成喹啉
收稿日期: 2020-07-01
修回日期: 2020-08-07
网络出版日期: 2020-08-31
基金资助
国家自然科学基金(21272006)
Copper-Catalyzed Three-Component Synthesis of Quinolines via
Oxidation and Aza-Diels-Alder Reaction
Received date: 2020-07-01
Revised date: 2020-08-07
Online published: 2020-08-31
Supported by
the National Natural Science Foundation of China(21272006)
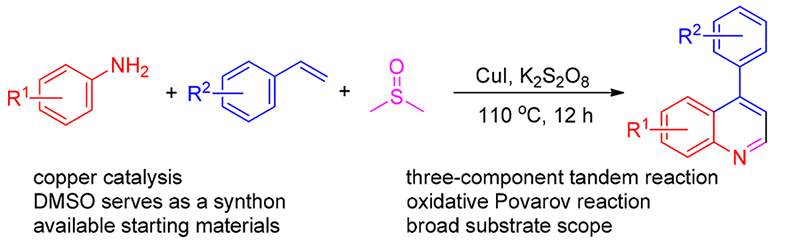
以苯胺、苯乙烯为原料, 二甲基亚砜(DMSO)为C1合成子, 开发了一种铜催化三组分反应合成喹啉衍生物的方法. 机理研究表明, 反应先形成亚胺中间体, 再发生Aza-Diels-Alder反应. 该方法具有高效、环境友好和底物适用范围广等特点.
关键词: 铜催化; 多组分反应; 喹啉衍生物; Aza-Diels-Alder反应
秦锋 , 汤琳 , 黄飞 , 李晓悦 , 张武 . 铜催化氧化和Aza-Diels-Alder反应三组分合成喹啉[J]. 有机化学, 2021 , 41(1) : 318 -324 . DOI: 10.6023/cjoc202007003
A tandem three-component reaction for the synthesis of quinolines from anilines, styrene and dimethyl sulfoxide (DMSO) has been developed. Dimethyl sulfoxide (DMSO) served as one-carbon synthon and solvent. The mechanism studies revealed that imine intermediate was involved and inverse electron demand Aza-Diels-Alder reaction was occurred. This method is featured by environmentally benign, good functional group tolerance and good to excellent yield.

| [1] | (a) Contelles M.; PrezMayoral J.E.; Samadi A.; Carreiras M.C. Chem. Rev. 2009, 109, 2652. |
| [1] | (b) Sridharan V.; Suryavanshi P.A.; Menndez J.C. Chem. Rev. 2011, 111, 7157. |
| [1] | (c) Renteria I.B.; Barranco P.G.; Garcia1, A.; Rivera, G.; Banik, K.Curr. Med. Chem. 2012, 19, 4377. |
| [1] | (d) Hu Y.Q.; Gao C.; Zhang S.; Xu L.; Xu Z.; Feng. L. S.; Wu, X.; Zhao, F.Eur. J. Med. Chem. 2017, 139, 222. |
| [1] | (e) Andrews S.; Burgess S.J.; Skaalrud D.; Kelly J.X.; Peyton D. J. Med. Chem. 2010, 53, 916. |
| [1] | (f) Tseng C.H.; Tung C.W.; Peng S.I.; Chen Y.L.; Tzeng C.C.; Cheng C.M. Molecules 2018, 23, 1036. |
| [1] | (g) Sandhya B.; Suresh K.; Sushma D.; Rajiv K. J. Pharm. Sci. 2010, 2, 64. |
| [1] | (h) Liberto N.A.; Simoes J.B.; Silva S.D.; Silva C.J.; Modolo L.V.; Fatima A. Bioorg. Med. Chem. 2017, 25, 1154. |
| [2] | (a) Cohn E.W. J. Am. Chem. Soc. 1939, 52, 3685. |
| [2] | (b) Gould R.G.; Jacobs W.A. J. Am. Chem. Soc. 1939, 61, 2890. |
| [2] | (c) Yamashkin S.A.; Oreshkina. E. A.Chem. Heterocycl. Compd. 2006, 42, 701. |
| [3] | (a) Cho C.S.; Kim B.T.; Kim T.J.; Shim S.C. Chem. Commun. 2001, 2576. |
| [3] | (b) Heravi M.M.; Asadi S.; Azarakhshi F. Curr. Org. Synth. 2014, 11, 701. |
| [4] | (a) Eisch J.J.; Dluzniewski T. J. Org. Chem. 1989, 54, 1269. |
| [4] | (b) Ren L.; Lei T.; Ye J.X.; Gong L.Z. Angew. Chem., Int. Ed. 2012, 51, 771. |
| [4] | (c) Perez-Mayoral M.; Musilova Z.; Gil B.; Marszalek B.; Polozij M. Dalton Trans. 2012, 41, 4036. |
| [4] | (d) Reddy B.V.; Venkateswarlu A.; Reddy G.N.; Reddy Y.V. Tetrahedron Lett. 2013, 54, 5767. |
| [5] | (a) Sloop J.C. J. Phys. Org. Chem. 2009, 22, 110. |
| [5] | (b) El Kharrat, S.; Laurent, P.; Blancou, H. Tetrahedron 2014, 70, 1252. |
| [5] | (c) Yuan S.Z.; Zhang K.H.; Xia J.J. Asian. J. Chem. 2013, 8, 5535. |
| [6] | (a) Ge´raldine M.; Claudia L.; Meryem B.; Guillaume D. Chem. Soc. Rev. 2013, 42, 902. |
| [6] | (b) Zhao P.; Wu X.; Zhou Y.; Geng X.; Wu Y.D.; Wu A.X. Org. Lett. 2019, 21, 2708. |
| [6] | (c) Devesh C.; Ankit K.D.; Rakesh K.; Upendra S. Eur. J. Org. Chem. 2019, 2019, 2753. |
| [7] | (a) Cheng Y.F.; Han X.S.; Ouyang H.C.; Rao Y. Chem. Commun. 2012, 48, 2906. |
| [7] | (b) Wang R.Z.; Fan H.J.; Zhao W.; Li F. Org. Lett. 2012, 18, 3558. |
| [7] | (c) Saunthwal R.K.; Patel M.; Verma A.K. J. Org. Chem. 2016, 81, 6563. |
| [7] | (d) Yao S.; Zhou K.J.; Wang J.B.; Cao H.G.; Yu L.; Wu J.Z.; Qiu P.H.; Xu Q. Green Chem. 2017, 19, 2945. |
| [8] | Huma H.Z.S.; Halder R.; Kalra S.S.; Das J.; Iqbal J. Tetrahedron Lett. 2002, 43, 6485. |
| [9] | Kong L.; Yu S.; Zhou X.; Li X. Org. Lett. 2016, 18, 588. |
| [10] | (a) Xu X.F.; Liu W.M.; Wang Z.Q.; Feng Y.Q.; Yan Y.L.; Zhang X. Tetrahedron Lett. 2016, 57, 226. |
| [10] | (b) Zhang X.; Xu X.F. Asian J. Chem. 2014, 9, 3089. |
| [11] | (a) Li C.S.; Li J.X.; An Y.N.; Peng J.W.; Wu W.Q.; Jiang H.F. J. Org. Chem. 2016, 81, 12189. |
| [11] | (b) Yuan J.; Yu J.T.; Jiang Y.; Cheng J. Org. Biomol. Chem. 2017, 15, 1334. |
| [11] | (c) Hu K.; Zheng Q.Q.; Gong J.L.; Cheng T.X.; Qi L.J.; Shao Y.L.; Chen J.X. Org. Lett. 2018, 20, 3083. |
| [12] | (a) Youn S.W.; Yoo H.J.; Lee E.M.; Lee S.Y. Adv. Synth. Catal. 2018, 360, 278. |
| [12] | (b) Phanindrudu M.; Wakade S.B.; Tiwari D.K.; Likhar P.R.; Tiwari D.K. J. Org. Chem. 2018, 83,9137. |
| [12] | (c) Jiang T.S.; Wang X.; Zhang X.L. Tetrahedron Lett. 2018, 59, 2979. |
| [13] | Midya S.P.; Sahoo M.K.; Landge V.; Rajamohanan P.R.; Balaraman E. Nat. Commun. 2015, 6, 8591. |
| [14] | (a) Mondal R.R.; Khamarui S.; Maiti D.K. ACS Omega 2016, 1, 251. |
| [14] | (b) Xu X.F.; Yang Y.R.; Chen X.; Zhang X.; Yi W. Org. Biomol. Chem. 2017, 15, 9061. |
| [15] | (a) Wu X.F.; Natte K. Adv. Synth. Catal. 2016, 358, 336. |
| [15] | (b) Wu X.; Zhang J.J.; Liu S.; Gao Q.H.; Wu A.X. Adv. Synth. Catal. 2016, 358, 218. |
| [15] | (c) Wakada S.B.; Tiwari D.K.; Ganesh P.S.K. P.; Phanindrudu, M.; Likhar, P.R.; Tiwari, D.K.Org. Lett. 2017, 19, 4948. |
| [16] | Xu X.F.; Yang Y.R.; Zhang X.; Yi W. Org. Lett. 2018, 20, 566. |
| [17] | (a) Jadhav S.D.; Singh A. Org. Lett. 2017, 19, 5673. |
| [17] | (b) Liu Y.F.; Hu Y.H.; Cao Z.Z.; Zhan X.; Luo W.P.; Liu Q.; Guo C.C. Adv. Synth. Catal. 2018, 360, 2691. |
| [18] | Cen J.C.; Li J.X.; Zhang Y.; Zhu Z.Z.; Yang S.R.; Jiang H.F. Org. Lett. 2018, 20, 4434. |
| [19] | Ramesh D.; Shekaraiah D.; Bhahwal A.S. Org. Chem. Front. 2015, 2, 515. |
| [20] | Liu J.; Liu F.; Zhu Y.Z.; Ma X.G.; Jia X.D. Org. Lett. 2015, 17, 1409. |
| [21] | (a) Ji X.C.; Huang H.W.; Li Y.B.; Chen H.J.; Jiang H.F. Angew. Chem., Int. Ed. 2012, 51, 7292. |
| [21] | (b) Wang H.K.; Wang C.; Huang K.M.; Liu L.Y.; Chang W.X.; Li J. Org. Lett. 2016, 18, 2367. |
/
| 〈 |
|
〉 |