

新型2,4,6,-取代嘧啶衍生物的设计、合成和抗肿瘤活性研究
收稿日期: 2020-07-29
修回日期: 2020-08-26
网络出版日期: 2020-08-31
基金资助
国家自然科学基金(81773562); 国家蛋白质研究项目(2018YFE0195100); 省部共建食管癌防治国家重点实验室资助的开放基金(K2020000X)
Design, Synthesis and Antitumor Activity Evaluation Research of Novel 2,4,6-Substituted Pyrimidine Derivatives
Received date: 2020-07-29
Revised date: 2020-08-26
Online published: 2020-08-31
Supported by
the National Natural Science Foundation of China(81773562); the National Key Research Program of Proteins(2018YFE0195100); the Openning Fund from State Key Laboratory of Esophageal Cancer Prevention & Treatment(K2020000X)
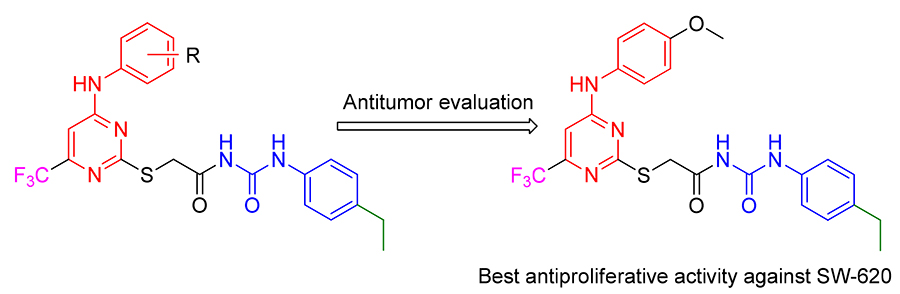
为了寻找高效的新型抗肿瘤药物, 设计合成了一系列2,4,6-取代嘧啶衍生物, 并使用噻唑蓝(MTT)法对4种人的肿瘤细胞人结肠癌细胞(SW-620)、人前列腺癌细胞(PC-3)、人非小细胞肺癌细胞(A549)和人胃癌细胞(MGC-803)进行了体外抗肿瘤活性研究. 其中化合物 N-((4-乙基苯基)氨基甲酰基)-2-((4-(对甲苯基氨基)-6-(三氟甲基)嘧啶-2-基)硫代)乙酰胺(5i), 2-((4-((4-乙氧基苯基)氨基)-6-(三氟甲基)嘧啶-2-基)硫基)- N-((4-乙基苯基)氨基甲酰基)乙酰胺(5o)和 N-((4-乙基苯基)氨基甲酰基)-2-((4-((4-甲氧基苯基)氨基)-6-(三氟甲基)嘧啶-2-基)硫代)乙酰胺(5r)对4种测试的癌细胞系显示出高的抗肿瘤增殖活性, 特别是化合物5r具有最高的抑制活性, 对SW-620的IC50值最低, 为1.46 μmol/L. 进一步机制研究表明, 化合物5r诱导SW-620凋亡, 使细胞周期阻滞在S期. 分子对接揭示了化合物5r可以很好地结合表皮生长因子受体(EGFR)的活性位点, 被认为是一种有前途的化合物, 可用于进一步研究开发新的抗癌药物.
张洋 , 张路野 , 王继宽 , 刘丽敏 , 王涛 , 栗娜 , 汪正捷 , 刘秀娟 , 陈雅欣 , 赵丹琳 , 郑甲信 , 单丽红 , 刘宏民 , 张秋荣 . 新型2,4,6,-取代嘧啶衍生物的设计、合成和抗肿瘤活性研究[J]. 有机化学, 2021 , 41(1) : 310 -317 . DOI: 10.6023/cjoc202007067
With the expectation to find out novel and effective anti-tumor agents, a series of novel 2,4,6-substituted pyrimidine derivatives were synthesized and evaluated for their anti-tumor activity against four human cancer cells [SW-620 (human colon cancer cells), PC-3 (human prostate cancer cells), A549 (Human non-small cell lung cancer cells), MGC-803 (human gastric cancer cells)] using methyl thiazolyl tetrazolium (MTT) assay. Among tested compounds, N-((4-ethylphenyl)- carbamoyl)-2-((4-( p-tolylamino)-6-(trifluoromethyl)pyrimidin-2-yl)thio)acetamide (5i), 2-((4-((4-ethoxyphenyl)amino)-6-(tri- fluoromethyl)pyrimidin-2-yl)thio)- N-((4-ethylphenyl)carbamoyl)acetamide (5o) and N-((4-ethylphenyl)carbamoyl)-2-((4-((4- methoxyphenyl)amino)-6-(trifluoromethyl)pyrimidin-2-yl)thio)acetamide (5r) displayed strong antiproliferative activity on 4 tested cancer cell lines. In particular, compound5r has the highest inhibitive activity, and possessed the lowest IC50 value of 1.46 μmol/L towards SW-620 cells. Further mechanism research shows that compound5rinduces SW-620 apoptosis, arrests cell cycle at S phase. Molecular docking reveals that5r can bind well to the active site of epidermal growth factor receptor (EG-FR), and may be considered as a promising compound amenable for further investigation for the development of new anticancer agents.

Key words: pyrimidine derivative; synthesis; antitumor activity; cell cycle; apoptosis
| [1] | Bray F.; Ferlay J.; Soerjomataram I.; Siegel R.L.; Torre L.A.; Jemal A. Ca-Cancer J. Clin.. 2018, 68, 394. |
| [2] | Woods D.; Turchi J.J. Cancer Biol. Ther. 2016, 14, 379. |
| [3] | Nurgali K.; Jagoe R.T.; Abalo R. Front. Pharmacol. 2018, 9, 245. |
| [4] | Ji Y.; Sun Q.; Zhang J.; Hu H. Biochem. Biophys. Res. Commun. 2018, 499, 719. |
| [5] | Zhang X.T.; Kang L.G.; Ding L.; Vranic S.; Gatalica Z.; Wang Z.Y. Oncogene 2011, 30, 770. |
| [6] | Lemmon M.A.; Schlessinger J. Cell 2010, 141, 1117. |
| [7] | Qu X.; Yang L.; Shi Q.; Wang X.; Wang D.; Wu G. Pathol., Res. Pract. 2018, 214, 1974. |
| [8] | Salomon D.S.; Brandt R.; Ciardiello F.; Normanno N. Crit. Rev. Oncol. Hematol. 1994, 19, 183. |
| [9] | Inoue A.; Kobayashi K.; Usui K.; Maemondo M.; Okinaga S.; Mikami I.; Ando M.; Yamazaki K.; Saijo Y.; Gemma A.; Miyazawa H.; Tanaka T.; Ikebuchi K.; Nukiwa T.; Morita S.; Hagiwara K. J. Clin. Oncol. 2009, 27, 1394. |
| [10] | Cohen M.H.; Williams G.A.; Sridhara R.; Chen G.; McGuinn W.D., Jr.; Morse, D.; Abraham, S.; Rahman, A.; Liang, C.; Lostritto, R.; Baird, A.; Pazdur, R. Clin. Cancer Res. 2004, 10, 1212. |
| [11] | Wada Y.; Iyoda M.; Matsumoto K.; Shindo-Hirai Y.; Kuno Y.; Yamamoto Y.; Suzuki T.; Saito T.; Iseri K.; Shibata T. PLoS One 2014, 9, 111728. |
| [12] | Wang X.K.; Huang L.Y.; Xu J.H.; Yang K.; Wang F.; Huang Z.C.; Ye S.; Fu L.W. Oncotarget 2014, 5, 11917. |
| [13] | Qin M.; Yan S.; Wang L.; Zhang H.; Zhao Y.; Wu S.; Gong P. Eur. J. Med. Chem. 2016, 115, 1. |
| [14] | Rekunge D.S., Khatri C.K., Chaturbhuj G.U. Tetrahedron Lett. 2017, 58, 4304. |
| [15] | Tokala R.; Bale S.; Janrao I.P.; Vennela A.; Kumar N.P.; Senwar K.R.; Shankaraiah N. Bioorg. Med. Chem. Lett. 2018, 28, 1919. |
| [16] | Chen J.N.; Wang X.F.; Li T.; Wu D.W.; Fu X.B.; Zhang G.J.; Shen X.C.; Wang H.S. Eur. J Med. Chem. 2016, 107, 12. |
| [17] | Keating G.M. Target. Oncol. 2017, 12, 243. |
| [18] | Clemence F.; Martret O.L.; Delevallee F.; Benzoni J.; Jouane A. J. Med. Chem. 1988, 31, 1453. |
| [19] | Plant N. Drug Discovery Today 2004, 9, 328. |
| [20] | Walter A.O.; Sjin R.T.; Haringsma H.J.; Ohashi K.; Sun J.; Lee K.; Dubrovskiy A.; Labenski M.; Zhu Z.; Wang Z.; Sheets M.; St Martin T.; Karp R.; van Kalken D.; Chaturvedi P.; Niu D.; Nacht M.; Petter R.C.; Westlin W.; Lin K.; Jaw-Tsai S.; Raponi M.; Van Dyke T.; Etter J.; Weaver Z.; Pao W.; Singh J.; Simmons A.D.; Harding T.C.; Allen A. Cancer Discovery 2013, 3, 1404. |
| [21] | Chen J.N.; Wang X.F.; Li T.; Wu D.W.; Fu X.B.; Zhang G.J.; Shen X.C.; Wang H.S. Eur. J. Med. Chem. 2016, 107, 12. |
| [22] | Xiao Z.; Zhou Z.; Chu C.; Zhang Q.; Zhou L.; Yang Z.; Li X.; Yu L.; Zheng P.; Xu S.; Zhu W. Eur. J. Med. Chem. 2020, 203, 112511. |
| [23] | Azimian F.; Hamzeh-Mivehroud M.; Shahbazi Mojarrad J.; Hemmati S.; Dastmalchi S. Eur. J. Med. Chem. 2020, 201, 112461. |
| [24] | Bajpai J.; Shrivastava P.K.; Khare R.K. J. Chemtracks 2010, 12, 369 .. |
| [25] | Abdel-Mohsen H.T.; Conrad J.; Harms K.; Nohr D.; Beifuss U. RSC Adv. 2017, 7, 17427. |
/
| 〈 |
|
〉 |