

N-氟代苯磺酰亚胺参与的过渡金属催化C—H氟化和胺化的研究进展
收稿日期: 2020-06-29
修回日期: 2020-08-11
网络出版日期: 2020-09-09
基金资助
宁波市自然科学基金(2019A610027); 陕西省教育厅专项科研计划(18JK0105)
Recent Advances in C—H Fluorination and Amination with N-Fluorobenzenesulfonimide
Received date: 2020-06-29
Revised date: 2020-08-11
Online published: 2020-09-09
Supported by
the Ningbo Municipal Natural Science Foundation(2019A610027); and the Education Foundation of Shaanxi Province(18JK0105)
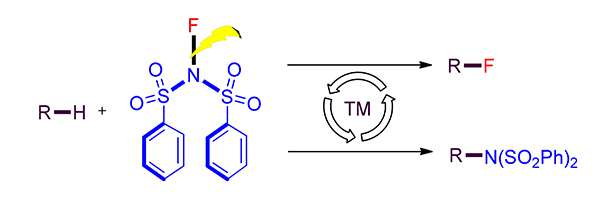
众多胺类及含氟化合物具有重要的生理活性, 在医药领域均具有不可替代的作用. 过渡金属催化的C—H胺化及氟化反应因其高反应效率及原子经济性, 受到了合成化学家的关注, 为生物碱类天然产物及含氟分子的合成提供了便利.N-氟代双苯磺酰胺(NFSI)兼有氟原子及含氮官能团, 可以在过渡金属催化下参与多种类型的有机反应, 实现C—H键的氟化或胺化. 因此, 探索NFSI参与的C—H键直接氟化或胺化反应具有重要意义.综述了近十年NFSI参与的C—H活化构建C—N键和C—F键方法的研究进展, 围绕各类方法的反应机理和应用范围进行阐述, 同时对该领域的局限性和发展前景进行总结和展望.
王威霖 , 陈卫东 , 骆钧飞 , 解攀 . N-氟代苯磺酰亚胺参与的过渡金属催化C—H氟化和胺化的研究进展[J]. 有机化学, 2021 , 41(2) : 543 -552 . DOI: 10.6023/cjoc202006069
The nitrogen- and fluorine-containing molecules display multiple important bioactivities which are crucial compounds in medicinal chemistry. The strategy relies on the transition-metal-catalyzed C—H amination and fluorination has received much attention due to its atom- and step-economy, providing an alternative to the synthesis of many natural alkaloids and fluorides.N-Fluorobenzenesulfonimide (NFSI) consists of the fluoride atom and the nitrogen-containing functionality, it is frequently used in the reactions based on transition-metal-catalyzed C—H activation to construct both C—N and C—F bonds. In this mini-review, the recent research advances in the formation of C—N and C—F bonds through transition-metal-catalyzed C—H with NSFI are reviewed. The reaction scopes and mechanisms are discussed in details, and the limitations of current procedures and the prospects for the future are summarized.

| [1] | (a) Liao G.; Wu Y.-J.; Shi B.-F. Acta Chim. Sinica 2020, 78, 289. (in Chinese) |
| [1] | 廖港, 吴勇杰, 史炳锋, 化学学报, 2020, 78, 289.). |
| [1] | (b) Zhao Q.; Meng G.; Nolan S.P.; Szostak M. Chem. Rev. 2020, 120, 1981. |
| [1] | (c) Mao P.; Zhu J.; Yuan J.; Yang L.; Xiao Y.; Zhang C. Chin. J. Org. Chem. 2019, 39, 1529. (in Chinese) |
| [1] | 毛璞, 朱军亮, 袁金伟, 杨亮茹, 肖咏梅, 张长森, 有机化学, 2019, 39, 1529.). |
| [1] | (d) Rej S.; Chatani N. Angew. Chem. Int. Ed. 2019, 58, 8304. |
| [1] | (e) Duarah G.; Kaishap P.P.; Begum T.; Gogoi S. Adv. Synth. Catal. 2019, 361, 654. |
| [1] | (f) Luo F. Chin. J. Org. Chem. 2019, 39, 3084. (in Chinese) |
| [1] | 罗飞华, 有机化学, 2019, 39, 3084.). |
| [1] | (g) Chu J. C. K.; Rovis T. Angew. Chem. Int. Ed. 2018, 57, 62. |
| [1] | (h) Wang S.; Yan F.; Wang L.; Zhu L. Chin. J. Org. Chem. 2018, 38, 291. (in Chinese) |
| [1] | 汪珊, 严沣, 汪连生, 朱磊, 有机化学, 2018, 38, 291.). |
| [2] | (a) Friis S.D.; Johansson M.J.; Ackermann L. Nat. Chem. 2020, 12, 511. |
| [2] | (b) Gruss H.; Sewald N. Chem.- Eur. J. 2020, 26, 5328. |
| [2] | (c) Zhang Y.; Shen S.; Fang H.; Xu T. Org. Lett. 2020, 22, 1244. |
| [2] | (d) Ren Q.; Nie B.; Zhang Y.; Zhang J. Chin. J. Org. Chem. 2018, 38, 2465. (in Chinese) |
| [2] | 任青云, 聂飚, 张英俊, 张霁, 有机化学, 2018, 38, 2465.). |
| [3] | (a) Felpin F.-X.; Sengupta S. Chem. Soc. Rev. 2019, 48, 1150. |
| [3] | (b) Xu P.; Duan X. Chin. J. Org. Chem. 2019, 39, 3315. (in Chinese) |
| [3] | 徐鹏, 段新红, 有机化学, 2019, 39, 3315.). |
| [3] | (c) Nareddy P.; Jordan F.; Szostak M. ACS Cat. 2017, 7, 5721. |
| [4] | (a) Das R.; Kapur M. Asian J. Org. Chem. 2018, 7, 1524. |
| [4] | (b) Luo J.; Xu X.; Zhao Y.; Liang H. Chin. J. Org. Chem. 2017, 37, 2873. (in Chinese) |
| [4] | 骆钧飞, 徐星, 赵延超, 梁洪泽, 有机化学, 2017, 37, 2873.). |
| [4] | (c) Liao G.; Shi B.-F. Acta Chim. Sinica 2015, 73, 1283. (in Chinese) |
| [4] | 廖港, 史炳锋, 化学学报, 2015, 73, 1283.). |
| [5] | (a) Szpera R.; Moseley D. F. J.; Smith L.B.; Sterling A.J. Gouverneur V. Angew. Chem. Int. Ed. 2019, 58, 14824. |
| [5] | (b) He J.; Lou S.; Xu D. Chin. J. Org. Chem. 2016, 36, 1218. (in Chinese) |
| [5] | 何将旗, 娄绍杰, 许丹倩, 有机化学, 2016, 36, 1218.). |
| [5] | (c) Liang T.; Neumann C.N.; Ritter T. Angew. Chem. Int. Ed. 2013, 52, 8214. |
| [6] | (a) Engle K.M.; Mei T.-S.; Wang X.; Yu J.-Q. Angew. Chem. Int. Ed. 2011, 50, 1478. |
| [6] | (b) Wang X.; Leow D.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 13864. |
| [6] | (c) Ball N.D.; Gary J.B.; Ye Y.; Sanford M.S. J. Am. Chem. Soc. 2011, 133, 7577. |
| [7] | (a) Rueda-Becerril M.; Sazepin C.C.; Leung J. C. T.; Okbinoglu T.; Kennepohl P.; Paquin J.-F.; Sammis G.M. J. Am. Chem. Soc. 2012, 134, 4026. |
| [7] | (b) Halperin S.D.; Fan H.; Chang S.; Martin R.E.; Britton R. Angew. Chem. Int. Ed. 2014, 53, 4690. |
| [7] | (c) Sibi M.P.; Landais Y. Angew. Chem. Int. Ed. 2013, 52, 3570. |
| [8] | (a) Sibbald P.A.; Rosewall C.F.; Swartz R.D.; Michael F.E. J. Am. Chem. Soc. 2009, 131, 15945. |
| [8] | (b) Weng S.-S.; Hsieh K.-Y.; Zeng Z.-J.; Zhang J.-W. Tetrahedron Lett. 2017, 58, 670. |
| [9] | (a) Qiu S.; Xu T.; Zhou J.; Guo Y.; Liu G. J. Am. Chem. Soc. 2010, 132, 2856. |
| [9] | (b) Zhang H.; Song Y.; Zhao J.; Zhang J.; Zhang Q. Angew. Chem. Int. Ed. 2014, 53, 11079. |
| [10] | (a) Zhang H.; Pu W.; Xiong T.; Li Y.; Zhou X.; Sun K.; Liu Q.; Zhang Q. Angew. Chem. Int. Ed. 2013, 52, 2529. |
| [10] | (b) Wang D.; Wang F.; Chen P.; Lin Z.; Liu G. Angew. Chem. Int. Ed. 2017, 56, 2054. |
| [11] | (a) Zhang B.; Studer A. Org. Lett. 2014, 16, 1790. |
| [11] | (b) Lei B.; Wang X.; Ma L.; Li Y.; Li Z. Org. Biomol. Chem. 2018, 16, 3109. |
| [12] | (a) Wang D.; Wu L.; Wang F.; Wan X.; Chen P.; Lin Z.; Liu G. J. Am. Chem. Soc. 2017, 139, 6811. |
| [12] | (b) Zheng G.; Sun J.; Liu Y.; Yang S.; Li Y.; Sun H.; Zhang Q. J. Org. Chem. 2017, 82, 12813. |
| [13] | (a) Ni Z.; Zhang Q.; Xiong T.; Zheng Y.; Li Y.; Zhang H.; Zhang J.; Liu Q. Angew. Chem. Int. Ed. 2012, 51, 1244. |
| [13] | (b) Zhang X.; Wu R.; Liu W.; Qian D.-W.; Yang J.; Jiang P.; Zheng Q.-Z. Org. Biomol. Chem. 2016, 14, 4789. |
| [13] | (c) Bao F.; Cao Y.; Liu W.; Zhu J. RSC Adv. 2019, 9, 27892. |
| [14] | Lou S.J.; Xu D.Q.; Xia A.B.; Wang Y.F.; Liu Y.K.; Du X.H.; Xu Z.Y. Chem. Commun. 2013, 49, 6218. |
| [15] | Lou S.J.; Xu D.Q.; Xu Z.Y. Angew. Chem. Int. Ed. 2014, 53, 10330. |
| [16] | Lou S.-J.; Chen Q.; Wang Y.-F.; Xu D.-Q.; Du X.-H.; He J.-Q.; Mao Y. -J. Xu, Z.-Y.ACS Catal. 2015, 5, 2846. |
| [17] | Ning X.-Q.; Lou S.-J.; Mao Y.-J.; Xu Z.-Y.; Xu D.-Q. Org. Lett. 2018, 20, 2445. |
| [18] | Testa C.; Gigot E.; Genc S.; Decreau R.; Roger J.; Hierso J.-C. Angew. Chem. Int. Ed. 2016, 55, 5555. |
| [19] | Testa C.; Roger J.; Scheib S.; Fleurat-Lessard P.; Hierso J.C. Adv. Synth. Catal. 2015, 357, 2913. |
| [20] | Ding Q.P.; Ye C.Q.; Pu S.Z.; Cao B.P. Tetrahedron 2014, 70, 409. |
| [21] | Chen C.; Wang C.; Zhang J.; and Zhao Y. J. Org. Chem. 2015, 80, 942. |
| [22] | Gutierrez D.A.; Lee W. -C. C.; Shen Y.; Li J.J. Tetrahedron Lett. 2016, 57, 5372. |
| [23] | Lee J.B.; Kang M.E.; Kim J.; Lee C.Y.; Kee J.-M.; Park K. Myung J.-U.; Hong S.Y. Chem. Commun.; 2017, 53, 10394. |
| [24] | Zhu Q.; Ji D.; Liang T.; Wang X.; Xu Y. Org. Lett. 2015, 17, 3798. |
| [25] | Mao Y.-J.; Lou S.-J.; Hao H.-Y.; Xu D.-Q. Angew. Chem. Int. Ed. 2018, 57, 14085. |
| [26] | Chen Y.-Q.; Singh S.; Wu Y.; Wang Z.; Hao W.; Verma P.; Qiao J.X.; Sunoj R.B.; Yu J.-Q. J. Am. Chem. Soc. 2020, 142, 9966. |
| [27] | Zhu R.Y.; Tanaka K.; Li G.C.; He J.; Fu H.Y.; Li S.H.; Yu J.-Q. J. Am. Chem. Soc. 2015, 137, 7067. |
| [28] | Zhang Q.; Yin X.S.; Chen K.; Zhang S.Q.; Shi B.-F. J. Am. Chem. Soc. 2015, 137, 8219. |
| [29] | Miao J.; Yang K.; Kurek M.; Ge H. Org. Lett. 2015, 17, 3738. |
| [30] | Park H.; Verma P.; Hong K.; Yu J.-Q. Nat. Chem. 2018, 10, 755. |
| [31] | McMurtrey K.B.; Racowski J.M.; Sanford M.S. Org. Lett. 2012, 14, 4094. |
| [32] | (a) Lu S.; Tian L.-L.; Cui T.-W.; Zhu Y.-S.; Zhu X.; Hao X.-Q.; Song M.-P. J. Org. Chem. 2018, 83, 13991. |
| [32] | (b) Haines B.E.; Kawakami T.; Kuwata K.; Murakami K.; Itami K.; Musaev D.G. Chem. Sci. 2017, 8. 988. |
| [32] | (c) Yin Y.; Xie J.; Huang F.-Q.; Qi L.-W.; Zhang B. Adv. Synth. Catal. 2017, 359, 1037. |
| [32] | (d) Barve B.D.; Wu Y.-C.; El-Shazly M.; Korinek M.; Cheng Y.-B.; Wang J.-J.; Chang F.-R. Tetrahedron. 2015, 71, 2290. |
| [32] | (e) Kawakami T.; Murakami K.; Itami K. J. Am. Chem. Soc. 2015, 137, 2460. |
| [32] | (f) Wang S.; Ni Z.; Huang X.; Wang J.; Pan Y. Org. Lett. 2014, 16, 5648. |
| [32] | (g) Wang X.; Lei B.; Ma L.; Jiao H.; Xing W.; Chen J.; Li Z. Adv. Synth. Catal. 2013, 359, 4284. |
| [33] | Tang R.-J.; Luo C.-P.; Yang L.; Li C.-J. Adv. Synth. Catal. 2013, 355, 869. |
| [34] | Dhiman A.K.; Gupta S.S.; Sharma R.; Kumar R.; Sharma U. J. Org. Chem. 2019, 84, 12871. |
| [35] | Iglesias A.; Alvarez R.; de Lera A.R.; Muniz K. Angew. Chem. Int. Ed. 2012, 51, 2225. |
| [36] | Jin L.; Zeng X.; Li S.; Hong X.; Qiu G. Liu. P.Chem. Commun. 2017, 53, 3986. |
| [37] | Dong Y.; Liu G. J. Org. Chem. 2017, 82, 3864. |
| [38] | Sun K.; Li Y.; Xiong T.; Zhang J.; Zhang Q. J. Am. Chem. Soc. 2011, 133, 1694. |
| [39] | Zhou Y.-F.; Zhu J.-M.; Li B.; Zhang Y.; Feng J.; Hall A.; Shi J.-Y.; Zhu W.-L. Org. Lett. 2016, 18, 3803. |
| [40] | Zheng Y.; Xiong T.; Lv Y.; Zhang J.; Zhang Q. Org. Biomol. Chem. 2013, 11, 7923. |
/
| 〈 |
|
〉 |