

水相中可见光下苯甲醇氧化合成喹唑啉酮衍生物的研究
收稿日期: 2020-07-09
修回日期: 2020-08-20
网络出版日期: 2020-09-16
基金资助
福建省自然科学基金(2016Y9052); 福建省自然科学基金(2016Y9053); 福建省自然科学基金(2017J01820); 福建省自然科学基金(FJNMP-201902); 福建省自然科学基金(2017-1-64)
Visible-Light-Induced Preparation of Quinazolinones by Oxidation of Benzyl Alcohols in Water
Received date: 2020-07-09
Revised date: 2020-08-20
Online published: 2020-09-16
Supported by
the Natural Science Foundation of Fujian Province(2016Y9052); the Natural Science Foundation of Fujian Province(2016Y9053); the Natural Science Foundation of Fujian Province(2017J01820); the Natural Science Foundation of Fujian Province(FJNMP-201902); the Natural Science Foundation of Fujian Province(2017-1-64)
张帆 , 侯慧青 , 许秀枝 , 陈志涛 , 柯方 . 水相中可见光下苯甲醇氧化合成喹唑啉酮衍生物的研究[J]. 有机化学, 2021 , 41(2) : 833 -841 . DOI: 10.6023/cjoc202007027
A novel visible-light-introduced reaction for the construction of quinazolinone derivatives via radical cyclization of 2-aminobenzamides with benzyl alochols under water phase has been developed. The reaction has been achieved in high yield under mild conditions by using I2 as photocatalyst, which is cheap, available and easy to handle. A variety of quinazolinones were obtained in yields up to 92%. It might provide a promising protocol for the synthesis of quinazolinone derivatives. Its application was performed by the synthesis of N-(2-fluoro-5-methylphenyl)-6-(2,2,2-trifluoroethoxy)pteridin-4-amine, which displayed significant inhibitory activity.
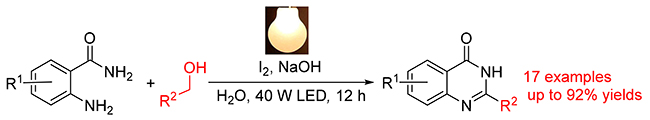
Key words: visible light; Iodine; quinazolinone; oxidant; catalyst
| [1] | (a) Kamble A.A.; Kamble R.R.; Chougala L.S.; Kadadevarmath J.S.; Maidur S.R.; Patil P.S.; Kumbar M.N.; Marganakop S.B. ChemistrySelect 2017, 2, 6882. |
| [1] | (b) Kamal A.; Bharathi E.V.; Reddy J.S.; Ramaiah M.J.; Dastagiri D.; Reddy M.K.; Viswanath A.; Reddy T.L.; Shaik T.B.; Pushpavalli S. N. C. V. L.; Bhadra M.P. Eur. J. Med. Chem. 2011, 46, 691. |
| [1] | (c) Chinigo G.M.; Paige M.; Grindrod S.; Hamel E.; Dakshanamurthy S.; Chruszcz M.; Minor W.; Brown M.L. J. Med. Chem. 2008, 51, 4620. |
| [1] | (d) Keopefly J.B.; Mead J.F.; Brokman J.A. J. Am. Chem. Soc. 1947, 69, 1837. |
| [1] | (e) Keopefly J.B.; Mead J.F.; Brokman J.A. J. Am. Chem. Soc. 1947, 69, 1048. |
| [2] | (a) Roopan S.M.; Khan F.N.; Jin J.S.; Kumar R.S. Res. Chem. Intermed. 2011, 37, 919. |
| [2] | (b) Hour M.J.; Huang L.J.; Kuo S.C.; Xia Y.; Bastow K.; Nakanishi Y.; Hamel E.; Lee K.H. J. Med. Chem. 2000, 43, 4479. |
| [2] | (c) Wang Z.W.; Wang M.X.; Yao X.; Li Y.; Tan J.; Wang L.Z.; Qiao W.T.; Geng Y.Q.;, Liu Y.X.; Wang Q.M. Eur. J. Med. Chem. 2012, 53, 275. |
| [2] | (d) Horton D.A.; Bourne G.T.; Smythe M.L. Chem. Rev. 2003, 103, 893. |
| [3] | (a) Huang W.; Liu J.; Wang C. Chin. J. Org. Chem. 2009, 29, 1533. (in Chinese) |
| [3] | 黄伟平, 刘建利, 王翠玲, 有机化学, 2009, 29, 1533.). |
| [3] | (b) Cagir A.; Jones S.H.; Gao R.; Eisenhauer B.M. J. Am. Chem. Soc. 2003, 125, 13628. |
| [4] | (a) Geng H.; Huang P.-Q. Chem. Rec. 2019, 19, 523. |
| [4] | (b) Wu J.-F.; Huang P.-Q. Chin. Chem. Lett. 2020, 31, 61. |
| [5] | (a) Vemula S.R.; Kumar D.; Cook G.R. ACS Catal. 2016, 6, 5295. |
| [5] | (b) Hrast M.; Ro?man K.; Juki? M.; Patin D.; Gobec S.; Sova M. Bioorg. Med. Chem. Lett. 2017, 27, 3529. |
| [5] | (c) Hase D.V.; Jayaram R.V.; Thirumalai K.; Swaminathan M. ChemistrySelect 2019, 4, 3440. |
| [5] | (d) Dabiri M.; Lehi N.F.; Movahed S.K.; Khavasi H.R. Eur. J. Org. Chem. 2019, 2933. |
| [5] | (e) Kumar D.; Vemula S.R.; Cook R. Green Chem. 2015, 17, 4300. |
| [6] | (a) Parashuram L.; Sreenivasa S.; Akshatha S.; Kumar V.U.; Kumar S. Asian J. Org. Chem. 2017, 6, 1755. |
| [6] | (b) Laha J.K.; Patel K.V.; Tummalapalli K. S. S; Dayal N. Chem. Commun. 2016, 52, 10245. |
| [6] | (c) Pariyar G.C.; Mitra B.; Mukherjee S.; Ghosh P. ChemistrySelect 2020, 5, 104. |
| [6] | (d) Kang H.; Wang W.; Sun Q.; Yang S.; Jin J.; Zhang X.; Ren X.; Zhang J.; Zhou J. Heterocycl. Commun. 2018, 24, 293. |
| [6] | (e) Rohokale R.S.; Kalshetti R.G.; Ramana C.V. J. Org. Chem. 2019, 84, 2951. |
| [7] | (a) Majumdar B.; Sarma D.; Jain S.; Sarma T.K. ACS Omega 2018, 3, 13711. |
| [7] | (b) Tang L.; Zhao X.; Zou G.; Zhou Y.; Yang X. Asian J. Org. Chem. 2016, 5, 335. |
| [7] | (c) Hakim Siddiki, S. M. A.; Kon, K.; Touchy, A.S.; Shimizu, K.-I. Catal. Sci. Technol. 2014, 4, 1716. |
| [7] | (d) Parua S.; Das S.; Sikari R.; Sinha S.; Paul N.D. J. Org. Chem. 2017, 82, 7165. |
| [8] | (a) Alam M.T.; Maiti S.; Mal P. Beilstein J. Org. Chem. 2018, 14, 2396. |
| [8] | (b) Palem J.D.; Alugubelli G.R.; Bantu R.; Nagarapu L.; Polepalli S.; Jain S.N.; Bathini R.; Manga V. Bioorg. Med. Chem. Lett. 2016, 26, 3014. |
| [8] | (c) Shang Y.-H.; Fan L.-Y.; Li X.-X.; Liu M.-X. Chin. Chem. Lett. 2015, 26, 1355. |
| [8] | (d) Hu B.-Q.; Wang L.-X.; Yang L.; Xiang J.-F.; Tang Y.-L. Eur. J. Org. Chem. 2015, 4504. |
| [8] | (e) Hisano T.; Ichikawa M.; Nakagawa A.; Tsuji M. Chem. Pharm. Bull. 1975, 23, 1910. |
| [8] | (f) Balakumar C.; Lamba P.; Pran Kishore D.; Lakshmi Narayana B.; Venkat Rao K.; Rajwinder K.; Raghuram Rao A.; Shireesha B.; Narsaiah B. Eur. J. Med. Chem. 2010, 45, 4904. |
| [8] | (g) Sharif M.; Opalach J.; Langer P.; Beller M.; Wu X. RSC Adv. 2014, 4, 8. |
| [8] | (h) Zhang Z.; Wang M.; Zhang C.; Zhang Z.; Lu J.; Wang F. Chem. Commun. 2015, 51, 9205. |
| [8] | (i) Jia F.-C.; Zhou Z.-W.; Xu C.; Wu Y.-D.; Wu A.-X. Org. Lett. 2016, 18, 2942. |
| [8] | (j) Abdel-Jalil R.J.; Voelter W.; Saeed M. Tetrahedron Lett. 2004, 45, 3475. |
| [9] | Seuli P.; Siuli D.; Rina S.; Suman S.; Nanda D.P. J. Org. Chem. 2017, 82, 7165. |
| [10] | Siuli D.; Suman S.; Deepannita S.; Rakesh M.; Gargi C.; Paula B.; Nanda D.P. J. Org. Chem. 2019, 84, 10160. |
| [11] | (a) Kalay E.; Kucukkececi H.; Kilic H.; Metin O. Chem. Commun. 2020, 56, 5901. |
| [11] | (b) Kim J.; Kang B.; Hong S.H. ACS Catal. 2020, 10, 6013. |
| [11] | (c) Ratushnyy M.; Kvasovs N.; Sarkar S.; Gevorgyan V. Angew. Chem., Int. Ed. 2020, 59, 10316. |
| [11] | (d) He W.-B.; Gao L.-Q.; Chen X.-J.; Wu Z.-L.; Huang Y.; Cao Z.; Xu X.-H.; He W.-M. Chin. Chem. Lett. 2020, 31, 1895. |
| [11] | (e) Xie L.-Y.; Bai Y.-S.; Xu X.-Q.; Peng X.; Tang H.-S.; Huang Y.; Lin Y. - W.; Cao Z.; He W.-M. Green Chem. 2020, 22, 1720. |
| [11] | (f) Xie L.-Y.; Liu Y.-S.; Ding H.-R.; Gong S.-F.; Tan J.-X.; He J. - Y.; Cao Z.; He W. - M.Chin. J. Catal. 2020, 41, 1168. |
| [11] | (g) Peng S.; Lin Y.; He W. Chin. J. Org. Chem. 2020, 40, 541. (in Chinese) |
| [11] | 彭莎, 林英武, 何卫民, 有机化学, 2020, 40, 541.). |
| [12] | Zhang M.-L.; Ruzi R.; Li N.; Xie J.; Zhu C. Org. Chem. Front. 2018, 5, 749. |
| [13] | (a) Yang J.; Fu T.; Long Y.; Zhou X.G. Chin. J. Org. Chem. 2017, 37, 1111. (in Chinese) |
| [13] | 杨军, 付婷, 龙洋, 周向葛, 有机化学, 2017, 37, 1111.). |
| [13] | (b) Li W.; Yin G.; Huang L.; Xiao Y.; Fu Z.; Xin X.; Liu F.; Li Z.; He W. Green Chem. 2016, 18, 4879. |
| [13] | (c) Luo F.H.; Long Y.; Li Z.K.; Zhou X.G. Acta Chim. Sinica 2016, 74, 805. (in Chinese) |
| [13] | 罗飞华, 龙洋, 李正凯, 周向葛, 化学学报, 2016, 74, 805.). |
| [13] | (d) Peng S.; Hu D.; Hu J.-L.; Lin Y.-W.; Tang S.-S.; Tang H.-S.; He J.-Y.; Cao Z.; He W.-M. Adv. Synth. Catal. 2019, 361, 5721. |
| [13] | (e) Zhang R.; Wang G.; Li H.; Duan G.; Wang K.; Xia C. Chin. J. Org. Chem. 2019, 39, 1429. (in Chinese) |
| [13] | 张瑞泽, 王国栋, 李洪爽, 段桂运, 王凯, 夏成才, 化学学报, 2019, 39, 1429.). |
| [13] | (f) Yue H.; Bao P.; Wang L.; Lv X.; Yang D. Chin. J. Org. Chem. 2019, 39, 463. (in Chinese) |
| [13] | 岳会兰, 鲍鹏丽, 王雷雷, 吕晓霞, 杨道山, 化学学报, 2019, 39, 463.). |
| [13] | (g) Peng S.; Song Y.-X.; He J.-Y.; Tang S.-S.; Tan J.-X.; Cao Z.; Lin Y.-W.; He W.-M. Chin. Chem. Lett. 2019, 30, 2287. |
| [14] | Zhong J.-J.; Meng Q.Y.; Liu B.; Li X.-B.; Gao X.-W.; Lei T.; Wu C.-J.; Li Z.-J.; Tung C.-H.; Wu L.-Z. Org. Lett. 2014, 16, 1988. |
| [15] | Ge W.; Zhu X.; Wei Y. RSC Adv. 2013, 3, 10817. |
| [16] | (a) Ke F.; Zhang P.; Xu Y.; Lin X.; Lin J.; Lin C.; Xu J. Synlett 2018, 29, 2722. |
| [16] | (b) Ke F.; Liu C.; Zhang P.; Xu J.; Chen X. Synth. Commun. 2018, 48, 3089. |
| [16] | (c) Ke F.; Xu Y.; Zhu S.; Lin X.; Lin C.; Zhou S.; Su H. Green Chem. 2019, 21, 4329. |
| [17] | (a) Hu Y.; Chen L.; Li B. RSC Adv. 2016, 6, 65196. |
| [17] | (b) Cao L.; Huo H.; Zeng H.; Yu Y.; Lu D.; Gong Y. Adv. Synth. Catal. 2018, 360, 4764. |
| [17] | (c) Nagasawa Y.; Matsusaki Y.; Nobuta T.; Tada N.; Miura T.; Itoh A. RSC Adv. 2015, 5, 63952. |
| [17] | (d) Wang Q.; Lv M.; Liu J.; Li Y.; Cao H.; Zhang X.; Xu Q. ChemSusChem 2019, 12, 3043. |
| [17] | (e) Liu Y.L.; Wang B.; Qiao X.F.; Tung C.-H.; Wang Y.F. ACS Catal. 2017, 7, 4093. |
| [17] | (f) Eva S.; Lisa-N. U.;, Phung, P. H. Q.; Gwen, S.; Frank, H.; Alexander, V.; Malte, B.Chem.- Eur. J. 2020, 26, 269. |
| [17] | (g) Yang J.Y.; Xie D.T.; Zhou H.Y.; Chen S.W.; Huo C.D.; Li Z. Org. Chem. Front. 2018, 5, 1325. |
| [17] | (h) Shang Y.-H.; Fan L.-Y.; Li X.-X.; Liu M.-X., Chin. Chem. Lett. 2015, 26, 1355. |
| [18] | (a) Doukas J.; Eide L.; Stebbins K.; Racanelli-Layton A.; Dellamary L.; Martin M.; Dneprovskaia E.; Noronha G.; Soll R.; Wrasidlo W.; Acevedo L.M.; Cheresh D.A. J. Pharmacol. Exp. Ther. 2009, 328, 758. |
| [18] | (b) Duan C.; Jia J.; Zhu R.; Wamg J. J. Heterocycl. Chem. 2012, 49, 865. |
| [18] | (c) Yang X.; Guan A. J. Fluorine Chem. 2014, 161, 1. |
| [19] | (a) Yu X.; Gao L.; Jia L.; Yamamoto Y.; Bao M. J. Org. Chem. 2018, 83, 10352. |
| [19] | (b) Abdullaha M.; Mohammed S.; Ali M.; Kumar A.; Vishwakarma R.A.; Bharate S.B. J. Org. Chem. 2019, 84, 5129. |
| [19] | (c) Upadhyaya K.; Thakur R.K.; Shukla S.K.; Tripathi R.P. J. Org. Chem. 2016, 81, 5046. |
| [19] | (d) Senadi G.C.; Kudale V.S.; Wang J.-J. Green Chem. 2019, 21, 979. |
| [19] | (e) Li F.; Lu L.; Liu P. Org. Lett. 2016, 18, 2580. |
| [19] | (f) Zheng Y.; Gao C.; Huang R.; Liu Y.; Xue Y.; An L. Asian J. Chem. 2016, 28, 95. |
| [19] | (g) Feng Y.; Li Y.; Cheng G.; Wang L.; Cui X. J. Org. Chem. 2015, 80, 7099. |
/
| 〈 |
|
〉 |